2023 Eye Drop Recall List: Brand by Brand Breakdown
Since February 2023, the FDA and various companies have been actively initiating recalls of eye drops and ointments due to concerns about potential bacterial contamination. The recalls, motivated by the risk of tainted products, have been steadily increasing, underscoring the ongoing commitment to consumer safety.
February 2023
It all started when Global Pharma Healthcare initiated a recall of its Artificial Tears Lubricant Eye Drops due to potential contamination. The recall encompasses all lots within their expiry period and impacts products distributed by EzriCare LLC and Delsam Pharma.
Artificial Tears Lubricant Eye Drops are designed to alleviate dryness and discomfort in the eyes resulting from minor irritations or exposure to wind and sun. Each ½ fl oz (15 mL) bottle, featuring a safety seal, contains 10 mg of carboxymethylcellulose sodium per 1 mL. The product is available from EzriCare (NDC 79503-0101-15, UPC 3 79503 10115 7) and Delsam Pharma (NDC 72570-121-15, UPC 72570-0121-15).
March 2023
Following Global Pharma Healthcare’s footsteps, Apotex Corp. voluntarily commenced a consumer-level recall for 6 lots of brimonidine tartrate ophthalmic solution, 0.15%. The distribution of these lots occurred nationwide in the United States from April 5, 2022, to February 22, 2023, under the guidance of the US FDA.
Brimonidine tartrate ophthalmic solution serves as an alpha-adrenergic receptor agonist, prescribed for the reduction of elevated intraocular pressure (IOP) in patients diagnosed with open-angle glaucoma or ocular hypertension.
One day after Apotex Corp., Pharmedica USA globally recalled two lots of Purely Soothing, 15% MSM Drops, at the consumer level due to concerns about non-sterility. The recall is prompted by the potential risk of eye infections and vision loss associated with the use of contaminated eye drops.
Purely Soothing's 15% MSM Drops are designed as an anti-inflammatory solution to alleviate symptoms of ocular irritation and swelling. The affected products come in white, cylindrical HDPE bottles with eye dropper caps and white lids.
Specifically, the recall encompasses LOT#: 2203PS01, 1 oz, UPC 7 31034 91379 9, and LOT#: 1808051, ½ oz, UPC 7 31034 91382 9. Consumers are strongly advised to check the lot numbers and product details to identify affected items and take appropriate precautions in response to the recall.
April 2023
Following its Chapter 7 bankruptcy filing on February 23, 2023, Akorn Operating Company LLC has halted all operations and dismissed employees at all its domestic US sites. The closure and discontinuation of Quality activities for marketed products have led the Akorn Trustee to initiate a voluntary recall of a range of within-expiry human and animal products.
● Apraclonidine Ophthalmic Solution 0.5%: It is primarily used to lower elevated intraocular pressure (IOP) in the eyes.
● Atropine Sulfate Ophthalmic Solution: It is a medication used in ophthalmology for specific eye-related purposes such as cycloplegia and mydriasis.
● Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment, 3.5g: It is an eye medication used to treat bacterial eye infections. The combination of bacitracin and polymyxin B works together to eliminate or inhibit the growth of susceptible bacteria that may cause eye infections.
● Cromolyn Sodium Ophthalmic Solution 4%: The drug is used as an ophthalmic solution to prevent and relieve symptoms of allergic conjunctivitis.
● Gonak Hypromellose Ophthalmic Solution: It is an ophthalmic solution used to protect the cornea during certain eye procedures.
● Ketorolac Tromethamine Ophthalmic Solution, 0.5%: Nonsteroidal anti-inflammatory drug (NSAID) for the temporary relief of ocular itching due to allergies.
● Levofloxacin Ophthalmic Solution: Eye drops for the treatment of bacterial eye infections.
● Moxifloxacin HCl Solution 0.5%: Ophthalmic solution used to treat bacterial eye infections.
● Neomycin & Polymyxin B Sulfates & Bacitracin Zinc Ophthalmic Ointment: Ophthalmic ointment used to treat eye infections.
● Olopatadine Solution 0.1% and 0.2%: Ophthalmic solution for the treatment of allergic conjunctivitis.
● Pilocarpine 1, 2, & 4%: Ophthalmic solution used to treat increased intraocular pressure in glaucoma.
● Proparacaine HCl Ophthalmic Solution, 0.5%: Ophthalmic anesthetic used during certain eye procedures.
● Sodium Chloride Ophthalmic Ointment: Ophthalmic ointment used for dry eyes and certain eye conditions.
● Timolol Maleate Ophthalmic Solution 0.5%, 2.5, 5, 10, & 15mL: Ophthalmic solution used to treat glaucoma and ocular hypertension.
● Tobramycin Ophthalmic Solution 0.3%: Ophthalmic solution used to treat eye infections.
● Tropicamide Ophthalmic Solution 0.5% and 1%: Ophthalmic solution used to dilate the pupils for eye examinations.
August 2023
The Pendopharm Division of Pharmascience initiated a recall for all 10 mL batches of Cromolyn Eye Drops due to the potential risk of microbial growth. The presence of microbes in a contaminated product poses a potential threat of eye infections, with heightened risks for vulnerable populations such as children, pregnant individuals, seniors, and those with weakened immune systems.
Cromolyn Eye Drops, which are available over-the-counter, are commonly used for the prevention and relief of symptoms related to seasonal allergic conjunctivitis, commonly known as pink eye.
Adding more fuel to the fire, the FDA cautioned consumers, urging them to refrain from buying and promptly cease the usage of two products: Dr. Berne's MSM Drops 5% Solution and LightEyez MSM Eye Drops – Eye Repair. This advisory stems from the possible existence of bacterial contamination, fungal contamination, or a combination thereof, within these products.
Dr. Berne’s Whole Health Products is the distributor of Dr. Berne’s items, while LightEyez Limited handles the distribution of LightEyez’ products.
Dr. Berne's MSM Drops 5% Solution and LightEyez MSM Eye Drops – Eye Repair are commonly used as eye care solutions.
October 2023
Joining the list of companies initiating recalls, Pine Pharmaceuticals issued two voluntary recalls, including repackaged Avastin and other products intended for ocular use. The company announced that there have been no reported adverse events linked to the affected products.
● Calcium Gluconate Oph 1% (500ML) Solution: Calcium Gluconate Ophthalmic Solution at 1% concentration is used to treat certain eye conditions.
● Tropi-Pen 1%-2.5% Ophthalmic Solution (5-15 mL): Tropi-Phen Ophthalmic Solution at 1%-2.5% concentration is used in ophthalmology. The specific ingredients may include an antimuscarinic agent and a phenylephrine, often used for pupil dilation.
● Lido-Phen 1%-1.5% (1ML) SDV: It is often used in ophthalmology. Lidocaine provides local anesthesia, and phenylephrine may be used for pupil dilation.
● Lidocaine HCL 4% (3 ML) Ophth: Lidocaine HCl Ophthalmic Solution at 4% concentration is a local anesthetic used in ophthalmic procedures to numb the eye.
After Pine Pharmaceuticals, the FDA has raised a red flag, advising against the purchase or use of 26 over-the-counter eye drop products. The concern centers around the potential risk of eye infections that could result in partial vision loss or even blindness.
The list of companies implicated in this warning comprises CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up&Up, and Velocity Pharma.
Here’s a list of recalled eye drops:
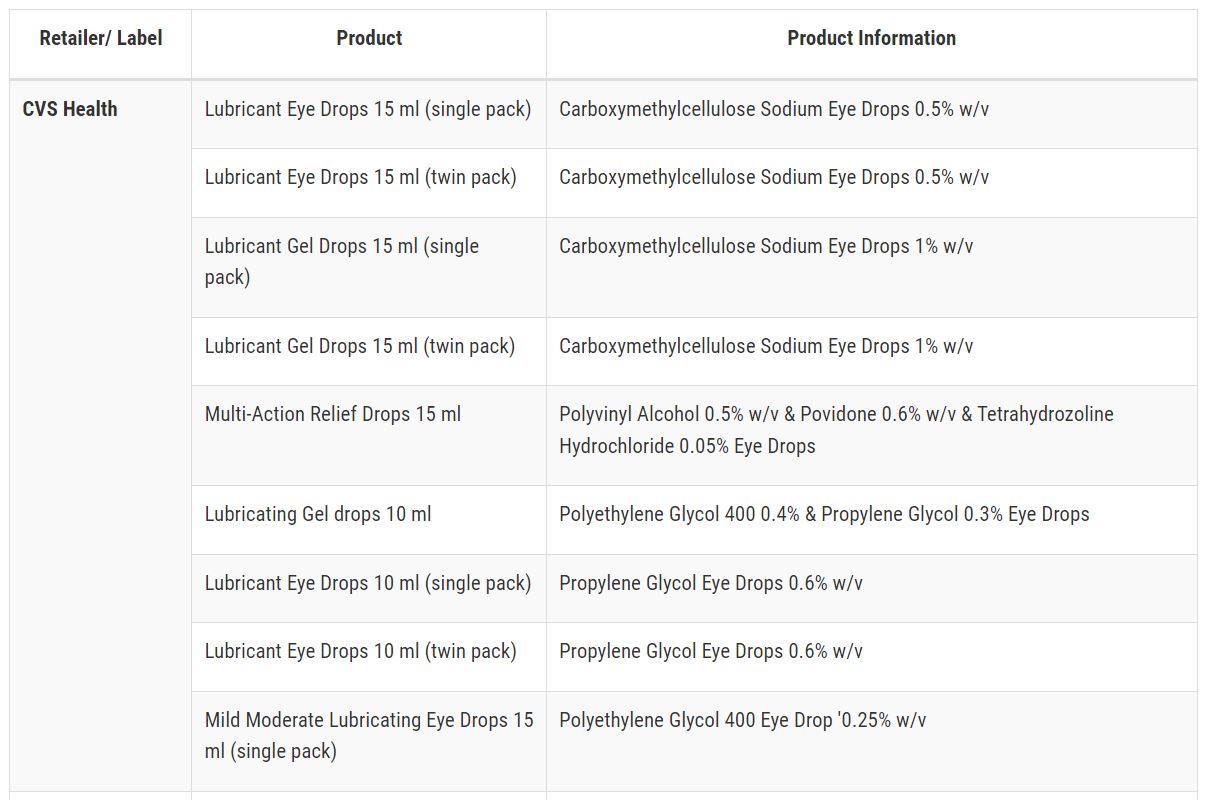
.jpg)
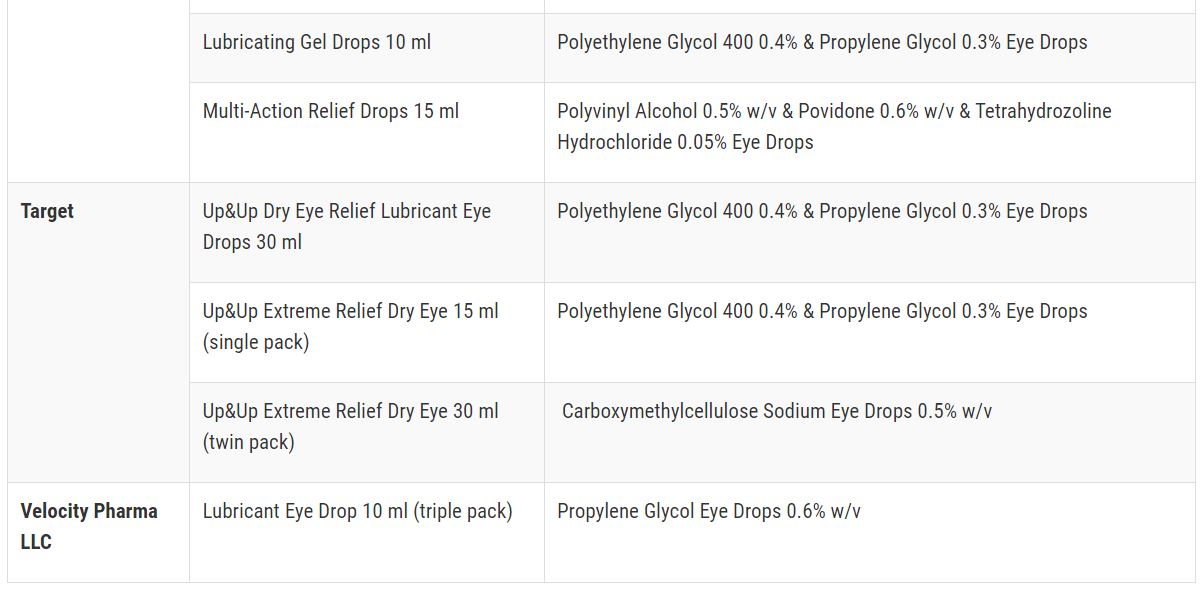
November 2023
In the most recent development, the US Food and Drug Administration's (FDA) Center for Drug Evaluation and Research (CDER) has taken a significant step by issuing a warning letter to the prominent e-commerce platform Amazon.com. The FDA's concerns revolve around the introduction of unapproved ophthalmic drug products into interstate commerce via Amazon's platform.
Among the identified products are Similasan Pink Eye Relief, The Goodbye Company Pink Eye, Can-C Eye Drops, Optique 1 Eye Drops, OcluMed Eye Drops, TRP Natural Eyes Floaters Relief, and Manzanilla Sophia Chamomile Herbal Eye Drops.
Summary
The recent surge in recalls of various eye drops has sent shockwaves throughout the entire ophthalmology industry, raising significant concerns and prompting heightened vigilance. Ophthalmologists and healthcare professionals are particularly alarmed by the situation, recognizing the critical importance of reliable and safe eye care products for patients.
This wave of recalls serves as a stark reminder of the necessity for stringent quality control measures, regulatory oversight, and transparent communication within the ophthalmology industry to ensure the well-being of patients and maintain the industry's reputation for delivering trustworthy eye care solutions.
(1).jpg)