2024 Recap: Top 8 FDA Approvals in Ophthalmology
The year 2024 has been a pivotal one for ophthalmology, with several novel FDA approvals introducing innovative treatments and technologies to the field. These breakthroughs address a wide range of conditions, from retinal diseases and glaucoma to presbyopia and surgical advancements. Each approval marks a step forward in improving patient care and expanding treatment options for eye health professionals.
Here is a list of the top 8 FDA approvals without any specific order.
1. Formycon and Klinge’s FYB203/AHZANTIVE®
.jpg)
Formycon AG and its licensing partner Klinge Biopharma GmbH have announced the FDA approval of FYB203/AHZANTIVE®, a biosimilar to Eylea®, for the treatment of serious retinal diseases.
The FDA has approved FYB203/AHZANTIVE® for the treatment of Age-Related Neovascular (wet) Macular Degeneration (nAMD) and other severe retinal conditions, including Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Macular Edema associated with Retinal Vein Occlusion (RVO).
In addition to the FDA approval, Formycon AG and Klinge Biopharma GmbH submitted a marketing authorization application for FYB203 to the European Medicines Agency (EMA) in late 2023. A decision from the EMA is expected by early 2025.
This FDA approval marks a significant milestone for FYB203/AHZANTIVE® and strengthens Formycon’s position in delivering advanced biosimilar solutions for retinal diseases.
2. Sandoz Receives FDA Approval for ENZEEVU™
.jpg)
Sandoz has received FDA approval for ENZEEVU™ (aflibercept-abzv), a biosimilar to EYLEA®, offering a cost-effective treatment option for various retinal conditions, including wet age-related macular degeneration (AMD) and diabetic retinopathy (DR). This milestone strengthens Sandoz’s U.S. biosimilar portfolio and reinforces its commitment to affordable, high-quality ophthalmic care.
Target Indications
The biosimilar is indicated for the treatment of:
• Wet AMD
• Diabetic Macular Edema (DME)
• Diabetic Retinopathy (DR)
• Macular Edema following Retinal Vein Occlusion (RVO)
The approval aligns with Sandoz’s strategy to expand its biosimilar portfolio and improve patient access to affordable medications, ensuring that cost-effective therapies are available for those who need them most. With this FDA approval, Sandoz takes another significant step in addressing the growing demand for affordable treatments in ophthalmology.
3. Glenmark’s Ophthalmic Solution for Glaucoma
.jpg)
Glenmark Pharmaceuticals has secured final FDA approval for its Brimonidine Tartrate and Timolol Maleate Ophthalmic Solution, 0.2%|0.5%, designed for glaucoma management. The solution has been approved as bioequivalent and therapeutically equivalent to Combigan® Ophthalmic Solution, 0.2%|0.5%, manufactured by AbbVie. Glenmark’s product will be distributed in the U.S. through Glenmark Pharmaceuticals Inc., USA.
The FDA’s approval confirms that Glenmark’s ophthalmic solution meets the stringent standards for safety and efficacy, offering healthcare providers and patients a reliable, cost-effective alternative to the established Combigan® product.
This milestone highlights Glenmark’s commitment to expanding access to high-quality, effective treatments in ophthalmology.
4. Biocon Biologics’ Yesafili™, Interchangeable Biosimilar Eylea
Biocon Biologics has announced FDA approval for YESAFILI™ (aflibercept-jbvf), an interchangeable biosimilar of EYLEA® (aflibercept). This marks a significant milestone as Biocon expands its footprint in the U.S. ophthalmology therapeutic market.
YESAFILI is a vascular endothelial growth factor (VEGF) inhibitor, designed to treat several serious retinal conditions:
• Neovascular (wet) Age-Related Macular Degeneration (AMD)
• Visual impairment due to macular edema secondary to retinal vein occlusion (branch RVO or central RVO)
• Visual impairment due to Diabetic Macular Edema (DME)
• Visual impairment due to Myopic Choroidal Neovascularization (myopic CNV)
YESAFILI demonstrates high similarity to its reference product, EYLEA®, with data confirming comparable quality, safety, and efficacy.
The FDA approval follows Biocon Biologics’ recent successes in Europe (September 2023) and the United Kingdom (November 2023), where YESAFILI was the first biosimilar aflibercept to gain approval. This U.S. approval highlights Biocon’s ongoing commitment to delivering high-quality, cost-effective treatments for retinal diseases, advancing care for millions of patients worldwide.
5. Caplin’s Ketoralac Tromethamine Ophthalmic Solution
.jpg)
Caplin Steriles Limited (Caplin), a subsidiary of Caplin Point Laboratories Limited, has received final approval from the USFDA for its Abbreviated New Drug Application (ANDA) for Ketorolac Tromethamine Ophthalmic Solution 0.5%, a generic equivalent to ACULAR Ophthalmic Solution by Allergan Inc.
Ketorolac Tromethamine Ophthalmic Solution 0.5% is a nonsteroidal anti-inflammatory drug (NSAID) indicated for:
• Treating post-cataract surgery inflammation.
• Providing temporary relief from ocular itching due to seasonal allergic conjunctivitis.
This approval is a significant milestone for Caplin Steriles, offering a cost-effective alternative to a trusted therapy. The product holds a substantial market presence, generating approximately $36 million in U.S. sales for the 12 months ending December 2023, according to IQVIATM.
By adding this FDA-approved product to its portfolio, Caplin enhances its position in the ophthalmic market while providing patients and healthcare providers with an affordable and effective option for managing ocular conditions.
6. FDA Approves Clobetasol Propionate Eye Drop
.jpg)
The U.S. Food and Drug Administration (FDA) has approved clobetasol propionate ophthalmic suspension 0.05% (APP13007), a groundbreaking therapy developed by Formosa Pharmaceuticals in collaboration with AimMax Therapeutics. This innovative treatment addresses postoperative inflammation and pain following eye surgeries, offering renewed hope to patients and healthcare providers.
This approval introduces the first FDA-approved ophthalmic clobetasol propionate solution, making it the first new steroid in the ophthalmic sector in over a decade. Leveraging the power of clobetasol propionate, a super-potent corticosteroid, the formulation is based on Formosa Pharma’s APNT nanoparticle platform, ensuring precision and effectiveness.
7. Bausch + Lomb Receives FDA Approval for Teneo Excimer Laser Platform
.jpg)
Bausch + Lomb has received FDA approval for the Teneo Excimer Laser Platform, designed for LASIK vision correction surgery targeting myopia and myopic astigmatism. The Teneo platform represents a significant advancement in excimer laser technology, emphasizing precision, efficiency, and usability to enhance outcomes for both surgeons and patients.
Key features of the Teneo include its exceptional accuracy and efficiency. The platform’s eye-tracker operates at 1,740Hz, three times the speed of the laser’s 500Hz repetition rate, ensuring precise ablation unaffected by eye movement. With an ablation time of approximately 1.2 seconds per diopter, it is the fastest excimer laser in the U.S. The customizable graphical touchscreen simplifies the surgical workflow, eliminating pre-treatment steps like nomogram use. Additionally, its compact 0.6m² footprint addresses space constraints in clinical settings.
Designed for surgeon and patient comfort, the Teneo features a 360-degree swiveling microscope and an adjustable treatment bed to accommodate patients of all sizes. The bed can swivel to integrate with secondary devices, minimizing patient movement during surgery. With this FDA approval, Bausch + Lomb aims to equip U.S. ophthalmologists with cutting-edge technology, setting a new standard for LASIK procedures.
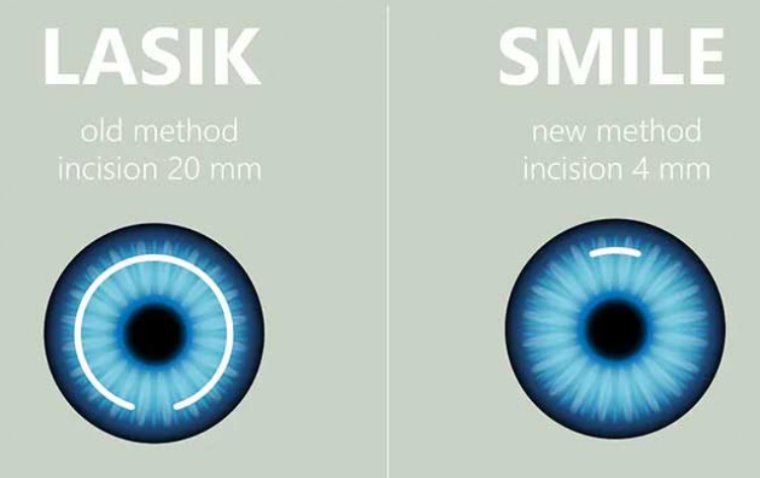
8. FDA Approves ZEISS' VISUMAX® 800 with SMILE® Pro Software
.jpg)
The FDA has approved the VISUMAX® 800, equipped with the SMILE® pro software from ZEISS, a cutting-edge femtosecond laser system designed for the surgical correction of nearsightedness with or without astigmatism.
This approval brings the VISUMAX® 800 to the U.S. market, building on the global success of SMILE technology, which has already treated over 8 million eyes worldwide, particularly in Asia and Europe. With the addition of the SMILE pro software, U.S. surgeons can now offer the latest in refractive technology, enhancing their practices and delivering exceptional patient outcomes.
The VISUMAX® 800 with SMILE® pro software delivers key advantages, including faster treatment times, as the lenticule is created in under 10 seconds thanks to its rapid 2 MHz laser pulse repetition rate. This improved efficiency minimizes stress for both surgeons and patients, enhancing the overall treatment experience.
A Promising Future for Ophthalmology
The 2024 FDA approvals highlight significant advancements in ophthalmology, addressing key medical needs and improving patient outcomes. From innovative biosimilars to cutting-edge surgical platforms, these breakthroughs are transforming the treatment of retinal diseases, glaucoma, presbyopia, and more.
As these therapies reach the market, they promise to enhance accessibility, affordability, and effectiveness, offering hope to millions worldwide. The future of ophthalmology is brighter than ever, driven by innovation and a commitment to preserving vision.
(1).jpg)
.jpg)