Bausch + Lomb Receives FDA Approval for Teneo Excimer Laser Platform
Bausch + Lomb has received the FDA approval for the Teneo Excimer Laser Platform for laser-assisted in situ keratomileusis (LASIK) vision correction surgery targeting myopia and myopic astigmatism.
Luc Bonnefoy, President of Global Surgical at Bausch + Lomb, expressed enthusiasm about the FDA approval: "Teneo has been well received and is widely adopted in more than 50 countries around the world, and now US ophthalmologists will benefit from this versatile laser. The precise engineering of this platform delivers a fast, small, technologically advanced machine that provides an exceptional experience for both surgeons and patients."
Read all about the Bausch + Lomb's TENEO™ Excimer Laser [2024] clinical trial results here
Features of Teneo Excimer Laser Platform
B+L highlights several distinctive features that set Teneo apart from previous excimer laser platforms.
1. Accuracy
The eye-tracker operates at an impressive 1,740Hz—over three times the speed of the laser's repetition. This speed ensures that the laser ablation pattern remains unaffected by a patient's eye movement, contributing to outstanding post-operative outcomes. The high-speed laser operates at 500Hz, making it the fastest ablation time among excimer lasers in the United States at approximately 1.2 seconds per diopter.
2. Efficiency
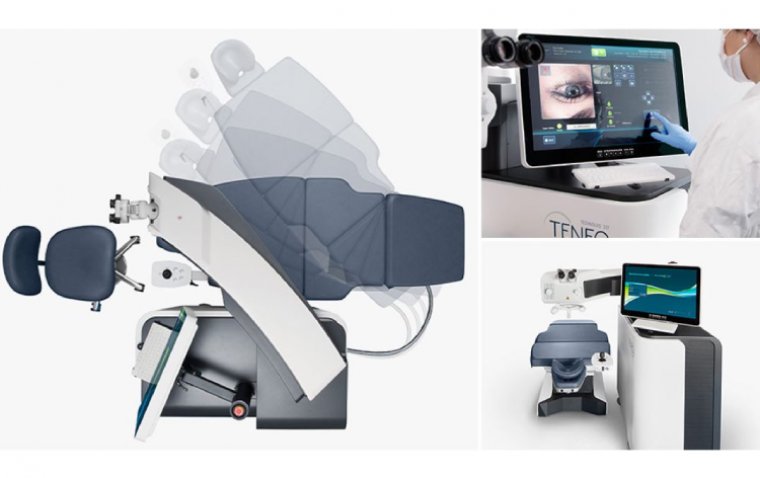
Teneo's customizable graphical user interface touchscreen simplifies setup and provides surgeons with easy access to patient data. The software treats the manifest refraction without requiring a nomogram, streamlining surgical planning by eliminating several pre-treatment steps. The intuitive process involves three simple steps: select the patient, choose and confirm treatment, and then treat.
Alongside time-saving benefits, Teneo's compact design (0.6m2 or 6.8 sq. ft) makes it the smallest excimer laser unit available in the United States, addressing concerns related to limited clinical space.
3. Usability
Teneo has been engineered for the comfort of both surgeons and patients. The 360-degree swiveling microscope adapts to surgeon's height and posture, enhancing comfort during procedures. The treatment bed accommodates patients of all sizes, swings out for easier access, and can be customized for optimal head positioning. The bed's swivel capability allows it to be positioned for a second treatment device, reducing the need for patients to move during the surgical process and enhancing overall comfort.
With the FDA approval, Bausch + Lomb aims to provide US ophthalmologists with an advanced tool that combines precision, efficiency, and usability, offering an exceptional LASIK experience for both medical professionals and patients.
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
Reference:
(1).jpg)