FDA Approves Genentech’s Vabysmo to Treat Wet AMD and DME
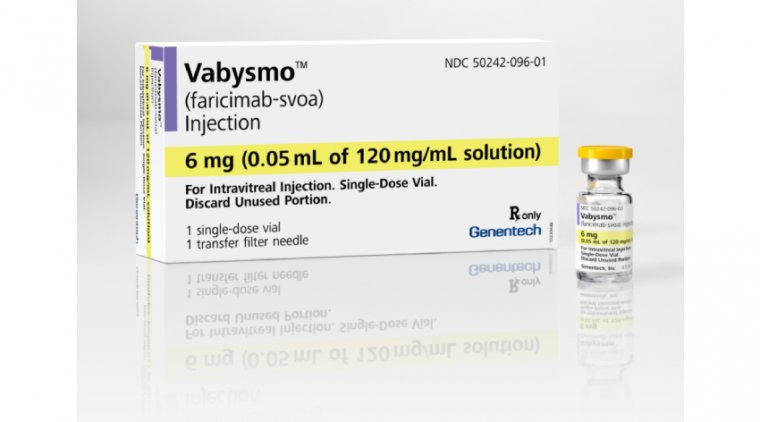
The FDA has approved Genentech's Vabysmo (faricimab-svoa) for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME), marking the first bispecific antibody approved for the eye.
Vabysmo is also the first and only FDA-approved injectable eye medicine for wet AMD and DME that improves and maintains vision with treatments from 1 to 4 months apart in the first year after four initial monthly doses, based on patient anatomy and vision outcomes.
Standard of care for wet AMD and DME typically requires eye injections every 1 to 2 months.
By neutralizing angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A), Vabysmo targets and inhibits two disease pathways connected to a number of vision-threatening retinal diseases.
“Vabysmo represents an important step forward for ophthalmology. It is the first bispecific antibody approved for the eye and a major advance in treating retinal conditions such as wet AMD and diabetic macular edema,” Charles Wykoff, MD, PhD, Director of Research at Retina Consultants of Texas in Houston and a Vabysmo phase 3 investigator, said in a company news release.
“With Vabysmo, we now have the opportunity to offer patients a medicine that could improve their vision, potentially lowering treatment burden with fewer injections over time.”
According to industry sources, Vabysmo costs $2,190 for each treatment. For patients who can go up to four months between treatments, the price of Vabysmo is $13,140 for the first year, including four initial monthly doses. Each year after, the annual cost reduces to $6,570.
The approval is based on positive results across four phase 3 studies in wet AMD and DME.
In the first year, patients treated with Vabysmo administered at intervals of up to 4 months had noninferior vision gains to those treated with aflibercept given every 2 months, according to the studies.
Vabysmo was generally well tolerated in all four studies, with a favorable benefit-risk profile. The most common adverse reaction (≥5%) reported in patients receiving Vabysmo was conjunctival hemorrhage (7%).
Two scientific papers and an editorial on these 1-year results were recently published in The Lancet.
Vabysmo is a medicine that works by blocking Ang-2 and VEGF-A pathways. Vision loss is believed to be caused by Ang-2 and VEGF-A disrupting blood vessels, which can lead to the formation of new leaky blood vessels and increased inflammation.
While further research is needed, preclinical studies have revealed that inhibiting both pathways has potentially complementary effects, such as stabilizing vessels and thereby lowering vascular leakage and inflammation.
“Vabysmo provides a new approach to treating vision-threatening retinal conditions through a mechanism of action that targets two pathways simultaneously,” said Levi Garraway, MD, PhD, chief medical officer and head of Global Product Development. “This is our second FDA approval in ophthalmology in recent months, underscoring our commitment to people living with retinal conditions.”
People with wet AMD have four monthly treatments with Vabysmo at first. They may undergo follow-up treatments every 2, 3, or 4 months, depending on anatomical and visual outcomes. DME patients initially receive four monthly treatments.
Following that, based on anatomical and eyesight outcomes, their treatment may be extended or reduced, with a range of 1 to 4 months between doses. A second approved treatment regimen for DME includes 6 monthly loading doses, followed by treatment every 2 months.
Although further efficacy was not proven in most patients given Vabysmo every month, some people with wet AMD and DME may be treated monthly if needed.
Vabysmo will be available in the United States in the coming weeks. The Vabysmo Marketing Authorization Application for the treatment of wet AMD and DME is also being evaluated by the European Medicines Agency.
(1).jpg)