ViaLase Laser Receives CE Mark Approval for Glaucoma Treatment
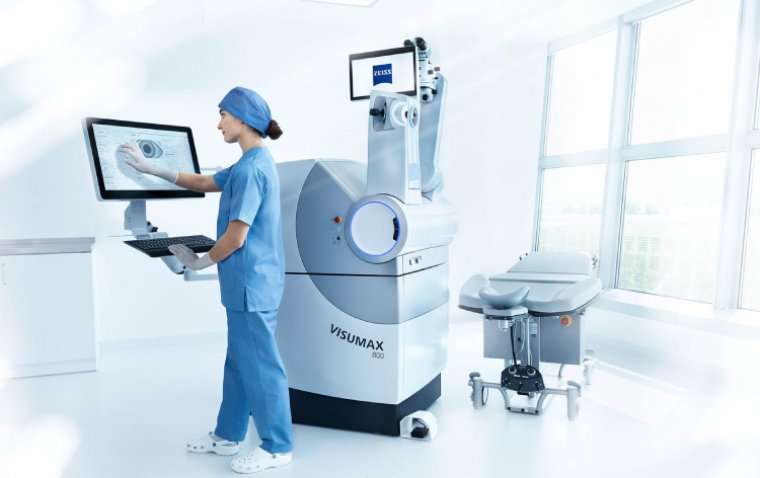
ViaLase has received CE Mark approval in the European Union for its ViaLase laser, designed to treat adult patients with primary open-angle glaucoma.
The ViaLase laser employs the precision of a femtosecond laser combined with micron-level image guidance to perform a noninvasive glaucoma treatment known as FLigHT—femtosecond laser, image-guided, high-precision trabeculotomy. This technology offers patients a non-pharmacologic, incision-free option for treating glaucoma.
In anticipation of commercialization in select European markets later this year, ViaLase has formed strategic distribution partnerships with Teleon Surgical for Germany and Austria, and Global Surgical Service for Spain and Portugal.
The ViaLase laser is not yet approved for use in the United States, the company noted.
Understanding Primary Open-Angle Glaucoma
Primary open-angle glaucoma (POAG) is the most common form of glaucoma, a group of eye conditions that damage the optic nerve, essential for good vision. This condition is characterized by a gradual increase in intraocular pressure due to the slow clogging of the drainage canals in the eye, which leads to optic nerve damage.
POAG often develops painlessly and without noticeable symptoms in its early stages, earning it the nickname "the silent thief of sight." If left untreated, it can lead to progressive, irreversible vision loss, starting with peripheral vision and eventually affecting central vision. Regular eye examinations are crucial for early detection and management of this potentially blinding disease.
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
(1).jpg)