FDA Approves Glenmark’s Ophthalmic Solution for Glaucoma, Bioequivalent to Abbvie's Combigan
Glenmark Pharmaceuticals has received final approval by the FDA for Brimonidine Tartrate and Timolol Maleate Ophthalmic Solution, 0.2%|0.5% for glaucoma treatment.
This solution has been recognized by the FDA as bioequivalent and therapeutically equivalent to Combigan® Ophthalmic Solution, 0.2%|0.5% by AbbVie. The product will be distributed in the U.S. by Glenmark Pharmaceuticals Inc., USA.
The FDA's approval confirms that Glenmark’s Brimonidine Tartrate and Timolol Maleate Ophthalmic Solution meets the necessary standards for safety and efficacy, making it a reliable alternative to the established Combigan® product. This offers an alternative option for patients and healthcare providers looking for effective treatments in ophthalmology.
According to IQVIA sales data, the Combigan® Ophthalmic Solution, 0.2%|0.5% market achieved annual sales of approximately $290.0 million for the 12-month period ending March 2024.
Glenmark's portfolio in the U.S. currently includes 196 products authorized for distribution and 51 Abbreviated New Drug Applications (ANDAs) awaiting approval. The company is also actively seeking external partnerships to enhance and accelerate the growth of their pipeline.
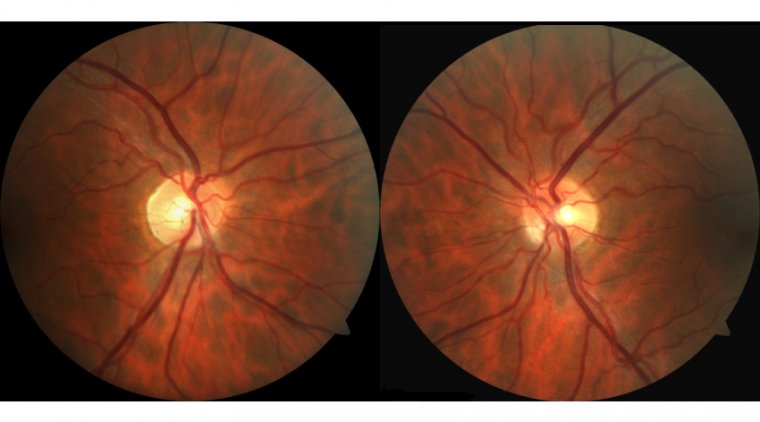
About Glaucoma
Glaucoma is a group of eye conditions that damage the optic nerve, often due to abnormally high pressure in the eye. It is one of the leading causes of blindness for people over the age of 60. The most common form, open-angle glaucoma, progresses slowly and painlessly, often without noticeable symptoms until significant vision loss has occurred. Another form, angle-closure glaucoma, can occur suddenly with symptoms such as severe eye pain, nausea, and blurred vision.
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
(1).jpg)