Pharmedica USA Recalls Purely Soothing 15% MSM Drops Due to Non-Sterility
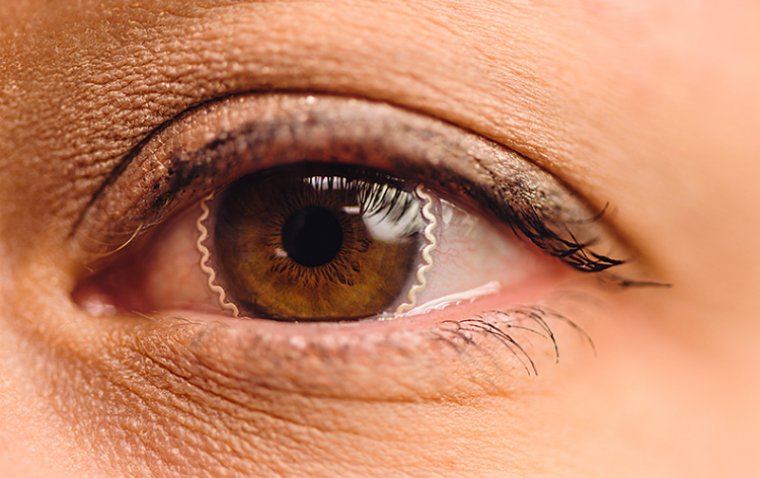
Pharmedica USA has issued a worldwide recall for two lots of Purely Soothing, 15% MSM Drops, at the consumer level due to non-sterility concerns. The use of contaminated eye drops can pose a risk of eye infections and vision loss.
The recall was prompted by the FDA, and at the time of the announcement, Pharmedica had not received any reports of adverse events or illness related to the recalled product. The eye drops were distributed globally by Purely Soothing via online e-commerce and trade shows, including Amazon Marketplace.
The FDA news release stated that Purely Soothing's 15% MSM Drops are intended for use as an anti-inflammatory to alleviate symptoms of ocular irritation and swelling. The drops are contained in white, cylindrical HDPE bottles with eye dropper caps and white lids. Specifically, the recall affects LOT#: 2203PS01, 1 oz, UPC 7 31034 91379 9; and LOT#: 1808051, ½ oz, UPC 7 31034 91382 9.
Pharmedica is advising customers to discontinue use of the product immediately and return it to the place of purchase. Wholesalers and retailers are also instructed to cease distribution and return the product to Pharmedica or provide evidence that it has been properly disposed of.
If consumers have questions regarding this recall, they may contact Pharmedica USA LLC by phone at 1-623-698-1752 or email at osm@pharmedicausa.com.
This marks the second such recall in a week. Earlier in the week, Apotex Corp. has also initiated a voluntary recall at the consumer level for 6 lots of brimonidine tartrate ophthalmic solution, 0.15%, distributed nationwide in the United States between April 5, 2022 to February 22, 2023 with the guidance of the US FDA.
(1).jpg)