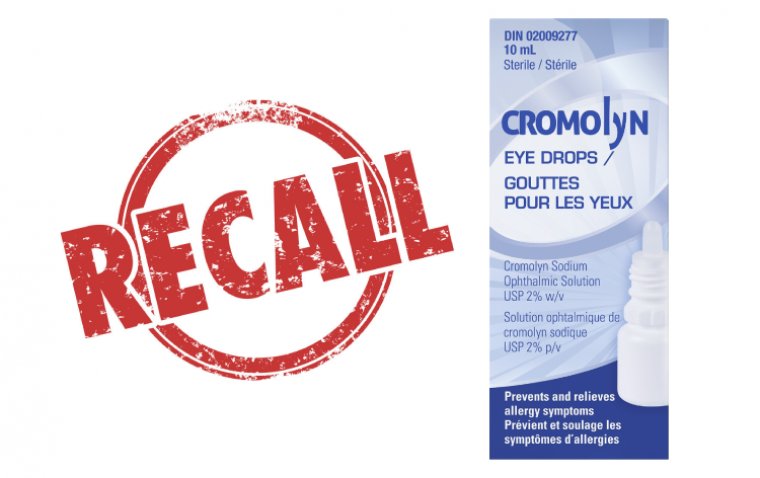
Health Canada Recalls Cromolyn Eye Drops Due to Infection Risks
The Pendopharm Division of Pharmascience is conducting a recall of all batches of the 10 mL Cromolyn Eye Drops due to the potential risk of microbial growth. The presence of microbes in a contaminated product could result in eye infections, particularly posing a higher risk to individuals such as children, pregnant individuals, seniors, and those with weakened immune systems who might be more susceptible to infections or complications arising from microbial contamination.
Cromolyn Eye Drops, available over-the-counter, are utilized to prevent and alleviate symptoms associated with seasonal allergic conjunctivitis (commonly referred to as pink eye) in adults and children aged five and above.
Through the company's testing, it was determined that the effectiveness of the preservative in the product might not be up to anticipated standards. This situation could potentially elevate the likelihood of microbial growth, including molds or bacteria, over time if introduced into the product.
For individuals in good health, the risk of significant eye infections due to contaminated eye drops remains relatively low, and any resultant infection might resolve on its own. In cases where the infection doesn't resolve naturally, topical antibiotics often provide effective treatment.
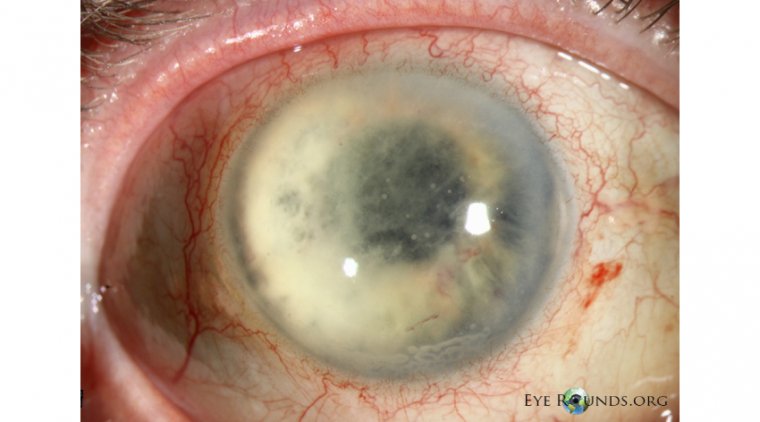
However, in rare instances, eye infections can lead to serious outcomes like vision loss, systemic infections, and even death, especially for those who are more prone to infections. Symptoms of infection could encompass eye pain, alterations in vision, sensitivity to light, eye redness, excessive discharge, and irregular pupils.
Although the potential growth of various microbes cannot be ruled out, testing revealed that the preservative may not effectively prevent the growth of a specific bacteria strain, known as Pseudomonas aeruginosa, if it infiltrates the product.
Alongside the aforementioned risks, individuals with compromised immune systems due to conditions like cystic fibrosis, HIV/AIDS, severe lung disorders, cancer, diabetes, or burns, should be particularly cautious. Pseudomonas aeruginosa can lead to severe infections such as pneumonia, bone infections, urinary tract infections, gastrointestinal infections, meningitis, and bloodstream infections.
What You Should Do
● Do not use the affected product. Return it to your local pharmacy for proper disposal.
● Consult a health care professional if you have used this product and have health concerns.
● Contact Pendopharm Division of Pharmascience Inc. by calling 1-888-550-6060 or emailing medinfo@pendopharm.com if you have questions about this recall.
● Report any health product-related side effects or complaints to Health Canada.
Reference
https://recalls-rappels.canada.ca/en/alert-recall/cromolyn-eye-drops-recalled-due-risk-infection
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now.
(1).jpg)