FDA Approves Continuous Delivery Ranibizumab Injection (Susvimo) for Diabetic Macular Edema
The U.S. Food and Drug Administration (FDA) has approved ranibizumab injection 100 mg/mL (Susvimo; Genentech) for the treatment of diabetic macular edema (DME). According to the company, Susvimo is the first and only FDA-approved therapy that maintains vision in DME patients with fewer treatments compared to standard intravitreal injections.
How Susvimo Works: Continuous Drug Delivery via Ocular Implant
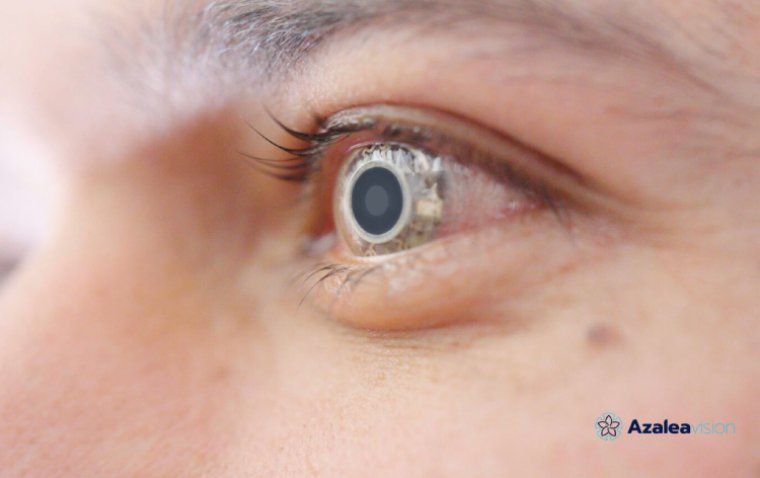
Susvimo is a refillable ocular implant designed for intravitreal use, providing a sustained-release formulation of ranibizumab. The implant is:
• Inserted through a one-time outpatient surgical procedure
• Continuously delivers ranibizumab over time
• Reduces the need for frequent intravitreal injections
Previously referred to as the Port Delivery System with ranibizumab (PDS) in the U.S., Susvimo offers a novel approach to treating DME by minimizing the burden of frequent injections.
Genentech’s Perspective on the Approval
Levi Garraway, MD, PhD, Chief Medical Officer and Head of Global Product Development at Genentech, emphasized the significance of this approval in a company press release:
"Susvimo presents a unique, convenient treatment alternative to routine eye injections for people with a potentially blinding diabetic eye condition. As the global prevalence of this condition continues to grow, today's FDA approval for Susvimo reflects our dedication to innovation and enhancing the patient experience."
Clinical Data Supporting FDA Approval
The FDA’s decision was based on positive one-year results from the Phase III PAGODA study (NCT04108156), a multicenter, randomized, active treatment-controlled, non-inferiority trial conducted in the U.S.
Key Findings from the PAGODA Study
• Enrolled 634 patients, randomized 3:2 to receive either:
• Susvimo refilled every six months
• Monthly intravitreal ranibizumab 0.5 mg injections
• Results demonstrated that Susvimo was non-inferior to monthly ranibizumab injections in terms of vision improvement.
• Patients receiving Susvimo maintained sustained vision benefits with a significantly lower treatment burden compared to those on standard monthly injections.
Availability and Previous FDA Approvals
Susvimo is now available to U.S. retina specialists and their patients for the treatment of DME. The therapy was first approved by the FDA in 2021 for the treatment of wet (neovascular) age-related macular degeneration (AMD).
Conclusion
The approval of Susvimo for DME marks a significant advancement in long-term treatment options for diabetic eye disease. By offering a continuous drug delivery system, Susvimo has the potential to enhance patient adherence, reduce treatment burden, and maintain vision more effectively than standard-of-care injections.
(1).jpg)