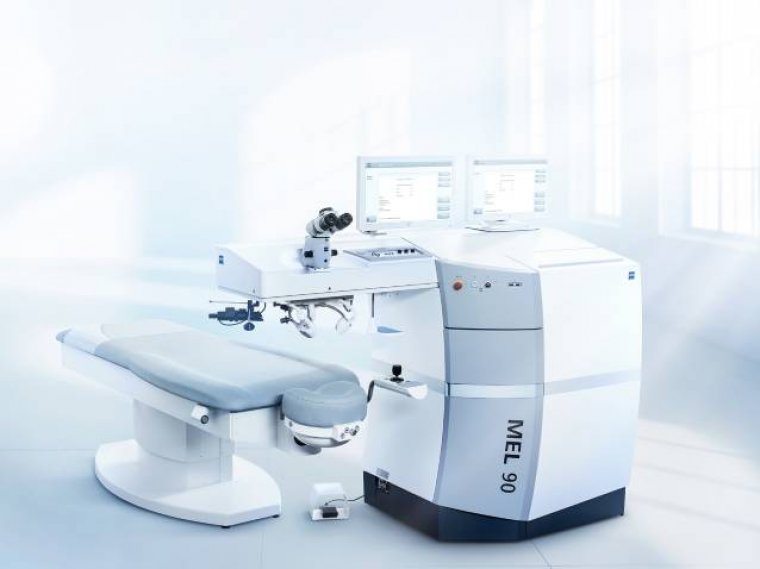
FDA Approves Zeiss MEL 90 Excimer Laser for Refractive Surgery
The FDA has officially approved the Zeiss MEL 90 excimer laser for all three major indications: myopia, hyperopia, and mixed astigmatism. This advanced laser technology is now available in the United States, offering a streamlined approach to refractive surgery by integrating seamlessly into the Corneal Refractive Workflow from Zeiss. The workflow complements the Visumax 800 and SMILE pro software, enabling surgeons to deliver optimized surgical outcomes.
A New Standard in Refractive Surgery
Andrew Chang, Head of Global Sales for ZEISS Medical Technology, expressed the company’s vision:
“ZEISS continues to break new ground as a leader in the LVC market, reflecting our ongoing commitment to and support of U.S. surgeons and patients with the latest refractive innovation that helps set practices apart and provides more options to more patients.”
With this FDA approval, the Zeiss MEL 90 enables surgeons to access an integrated refractive technology workflow, broadening patient reach and enhancing clinical outcomes.
Features of the Zeiss MEL 90 Excimer Laser
The MEL 90 excimer laser includes a range of innovative features, making it a game-changer for refractive surgery:
• Triple-A Ablation Algorithm:
A single ablation profile for sphero-cylindrical corrections, offering:
• High accuracy
• Reproducibility
• Predictability
• Tissue-saving ablation
• Fast Ablation Speed:
Ablates 1 diopter in just 1.3 seconds (optical zone: 6 mm) at 500 Hz, significantly reducing procedure time during LASIK for myopia.
• Active Eye Tracker:
Improves response time for enhanced treatment safety and stable results.
Customizable Options
The MEL 90 excimer laser allows for personalization to meet the preferences of surgeons and their teams:
• Adjustable touch screen positioning for better surgical space ergonomics.
• Option to add a second touch screen for optimized workflows.
• Additional connection features like an HD video port, network printer connection, and PDF export for seamless integration into practice operations.
Expert Opinions on the MEL 90
Dr. John Doane, a refractive surgeon at Discover Vision Centers in Kansas City, Missouri, praised the technology:
“The FDA approval of the ZEISS MEL 90 excimer laser is a game changer for refractive surgery in the U.S. This advanced technology, with its Triple-A ablation algorithm and fast ablation speed, sets a new benchmark for precision, safety, and efficiency.”
He also highlighted the laser’s ability to simplify treatment planning while ensuring predictable, tissue-conserving results. The integration of the MEL 90 with the ZEISS Visumax 800 femtosecond laser offers surgeons a streamlined workflow and superior patient outcomes.
Transforming Refractive Surgery
Magnus Reibenspiess, Head of Strategic Business Unit Ophthalmology at ZEISS Medical Technology, emphasized the impact of the MEL 90:
“The increasing global adoption of laser vision correction reflects the advancements and positive impact the technology continues to have on the quality of life for patients. With the integration of the ZEISS MEL 90, surgeons can confidently care for their patients with greater workflow efficiency and performance with enhanced outcomes.”
Conclusion
The FDA approval of the Zeiss MEL 90 excimer laser marks a significant milestone in refractive surgery. By combining advanced ablation technology with customizable features and an integrated workflow, the MEL 90 sets a new standard for precision, safety, and efficiency in treating myopia, hyperopia, and mixed astigmatism. This innovation opens new opportunities for surgeons to provide excellent outcomes while expanding patient access to cutting-edge refractive care.
Reference:
(1).jpg)