Large-Scale Study Provides New Insights into Rare Eye Disorders
Researchers have analyzed image and genomic data from the UK Biobank to find insights into rare diseases of the human eye. These include retinal dystrophies – a group of inherited disorders affecting the retina – which are also the leading cause of blindness certification in working-age adults.
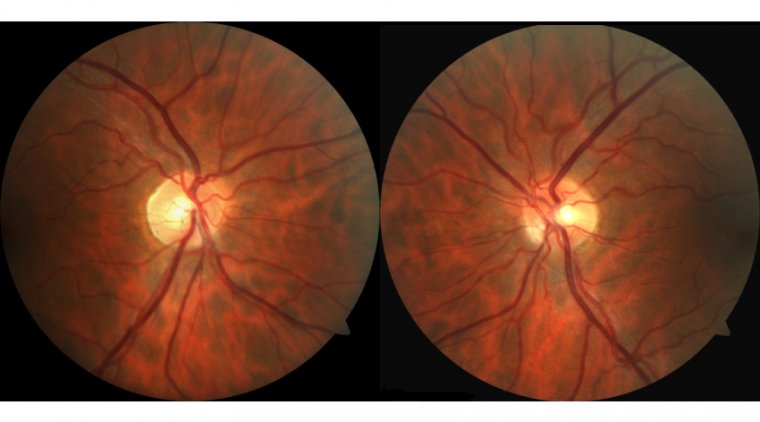
The researchers conducted a study, published in the journal PLOS Genetics, which focused on photoreceptor cells (PRCs) - the light-detecting cells present in the retina. Optical coherence tomography (OCT), a non-invasive imaging service commonly offered by opticians, was used to obtain images of these cells. By combining the OCT image data with genomic data stored in the UK Biobank, the researchers were able to conduct the largest genome-wide association study of PRCs to date.
Rare Retinal Dystrophies
Inherited mutations in genes expressed by photoreceptor cells (PRCs) are a frequent cause of rare diseases of the retina. Such mutations can cause the retina to function incorrectly, leading to vision impairment or even blindness. Although these diseases are individually rare, they are collectively the primary cause of blindness in working-age adults.
"We had access to coupled images and genotype data at a scale that had not been seen in a study of this kind," said Hannah Currant, former Ph.D. student at EMBL's European Bioinformatics Institute (EMBL-EBI) and Postdoctoral Fellow at the Novo Nordisk Foundation Center for Protein Research (CPR) University of Copenhagen. "Access to this enormous amount of data was critical to the study and enabled us to identify genetic links to rare retinal dystrophies. This work has identified new avenues for research and generated new questions about rare retinal dystrophies."
Linking Genotype and Phenotype
Optical coherence tomography (OCT) produces high-resolution images that can be used to identify different layers and structures within the retina. These images are frequently utilized in clinical settings to facilitate the diagnosis of eye disorders. In the present study, the researchers leveraged OCT images in combination with genomic and medical data of over 30,000 participants stored in the UK Biobank.
"The UK Biobank is a rich, invaluable resource that has enormous potential to enable genomic medicine," said Ewan Birney, Deputy Director General of the European Molecular Biology Laboratory (EMBL). "There is so much potential just waiting to be released from the data stored there, which lets us both understand human biology and how and when it goes wrong in disease."
Driving Genomic Medicine
The researchers performed genome-wide association studies (GWAS) on the UK Biobank data to identify genetic variations that are linked to variations in the thickness of the layers of photoreceptor cells (PRCs). This analysis allowed them to identify genomic variations that were associated with the thickness of one or more of the PRC layers, including those previously linked with known eye diseases. The newly identified genomic associations have been deposited and are publicly available through the GWAS Catalog.
While some of the identified genetic variants had already been linked to eye diseases, the study also found that several common genetic variants were located close to genes known to cause rare genetic eye diseases when disrupted. Notably, in one instance, the researchers were able to examine how common variants close to genes implicated in rare eye diseases affected the structure of the retina. This finding enhances our understanding of how specific common variants may impact these rare diseases, offering greater confidence when exploring rare disease collections.
"Systematic bioinformatic analysis of large-scale participant data cohorts is driving the future of genomic medicine," said Omar Mahroo, Professor of Retinal Neuroscience at University College London and Consultant Ophthalmologist at Moorfields Eye Hospital. "Having access to these data and being able to make these connections between disease phenotypes and genetic variation will open many new opportunities for modern disease diagnosis and therapeutics."
Reference: Hannah Currant et al, Sub-cellular level resolution of common genetic variation in the photoreceptor layer identifies continuum between rare disease and common variation, PLOS Genetics (2023). DOI: 10.1371/journal.pgen.1010587
(1).jpg)