2023 Recap: Top 12 Novel Ophthalmic Drugs That Received FDA Approval
2024 Recap is here! Click to review:
Top 8 FDA Approvals in Opthalmology
In a series of groundbreaking developments, the FDA has approved a range of innovative drugs in 2023, heralding a new era in ophthalmic treatments. Here's an in-depth look at each of these transformative medications.
*The listicle was organized chronologically.
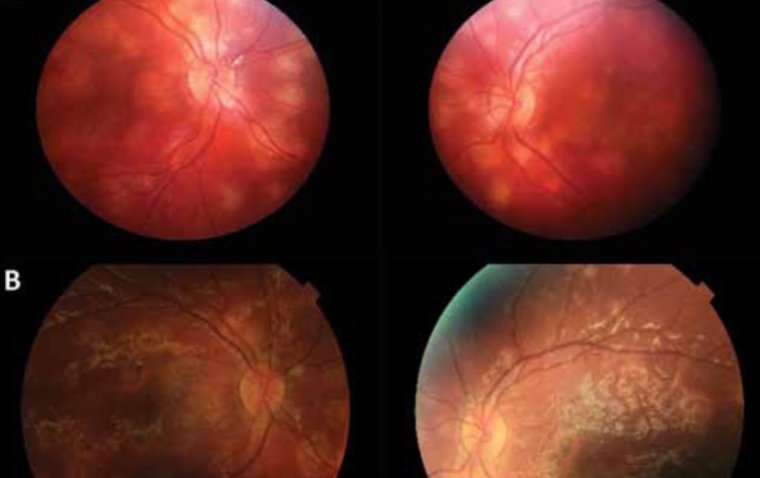
1. Apellis’ Syfovre for Geographic Atrophy:
.jpg) In February 2023, Apellis Pharmaceuticals' Syfovre (pegcetacoplan) received FDA approval for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). This approval is a historic milestone as it represents the first FDA-approved treatment option for patients with GA.
In February 2023, Apellis Pharmaceuticals' Syfovre (pegcetacoplan) received FDA approval for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). This approval is a historic milestone as it represents the first FDA-approved treatment option for patients with GA.
Currently, the European Medicines Agency is in the process of reviewing a marketing authorization application for Syfovre, with an expected decision in early 2024. Amarketing application for Syfovre has also been submitted to Health Canada.
Discover SYFOVRE, the first and only FDA-approved treatment for geographic atrophy.
2. Regeneron's EYLEA® Injection for Retinopathy of Prematurity:
.jpg) In February 2023, Regeneron Pharmaceuticals revealed that the FDA approved the use of EYLEA® (aflibercept) Injection as a treatment option for preterm infants with retinopathy of prematurity (ROP). This approval represents the first instance of EYLEA receiving approval for pediatric use and extends its indications to include five distinct retinal conditions caused by ocular angiogenesis.
In February 2023, Regeneron Pharmaceuticals revealed that the FDA approved the use of EYLEA® (aflibercept) Injection as a treatment option for preterm infants with retinopathy of prematurity (ROP). This approval represents the first instance of EYLEA receiving approval for pediatric use and extends its indications to include five distinct retinal conditions caused by ocular angiogenesis.
EYLEA® (aflibercept) Injection 2 mg (0.05 mL) is a prescribed medication authorized for treating patients with Wet Age-related Macular Degeneration (AMD), Macular Edema following Retinal Vein Occlusion (RVO), Diabetic Macular Edema (DME), Diabetic Retinopathy (DR), and Retinopathy of Prematurity (ROP).
Discover EYLEA®, the first pharmacologic treatment for retinopathy of prematurity approved by the FDA.
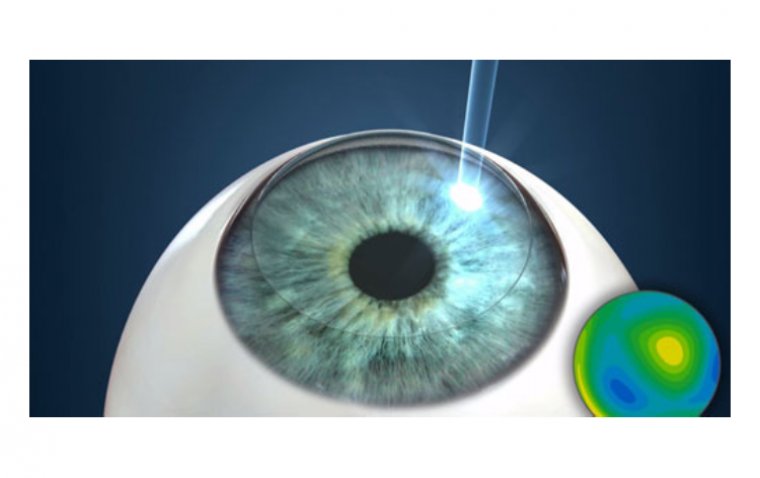
3. Allergan’s Vuity for Twice-Daily Dosing for Presbyopia
.jpg) In March 2023, Allergan announced that the FDA approved the use of Vuity (pilocarpine HCl ophthalmic solution) 1.25% twice a day for adults experiencing presbyopia. The second dose, comprising an extra drop in each eye, can be given 3-6 hours after the initial dose. This approval for twice-daily dosing of Vuity has the potential to extend its effectiveness for up to 9 hours.
In March 2023, Allergan announced that the FDA approved the use of Vuity (pilocarpine HCl ophthalmic solution) 1.25% twice a day for adults experiencing presbyopia. The second dose, comprising an extra drop in each eye, can be given 3-6 hours after the initial dose. This approval for twice-daily dosing of Vuity has the potential to extend its effectiveness for up to 9 hours.
Vuity obtained FDA approval for once-daily dosing in October 2021.
Discover VUITY's FDA-approved twice-daily dosing for adults with presbyopia.
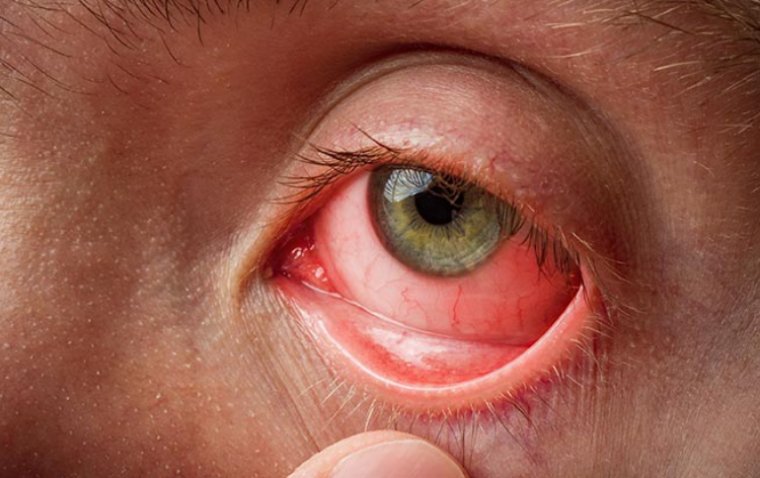
4. Bausch & Lomb and Novaliq's Miebo for DED:
.jpg)
In May 2023, Miebo, a collaboration between Bausch & Lomb and Novaliq, received FDA approval for Miebo (perfluorohexyloctane ophthalmic solution; formerly NOV03) for the treatment of the signs and symptoms of dry eye disease (DED).
Miebo is the first and only FDA-approved drug for DED specifically designed to address tear evaporation. The administration of Miebo involves applying a single drop to each eye four times a day, with the objective of reducing tear evaporation on the eye's surface.
Find out about the FDA's approval of Miebo by Bausch + Lomb and Novaliq, a new treatment option for dry eye.
5. Eyenovia's MydCombi for Mydriasis:
In May 2023, Eyenovia achieved a significant milestone with the FDA approval of MydCombi, the first ophthalmic spray designed for mydriasis. Mydcombi is a micro-dose ophthalmic spray containing tropicamide 1% and phenylephrine 2.5%.
This marks the first approval of a fixed-dose combination of tropicamide and phenylephrine in the United States, and it is also the first to use Eyenovia's state-of-the-art Optejet device to receive regulatory approval.
Learn about Mydcombi, the first ophthalmic spray for mydriasis by Eyenovia.
6. Novaliq's VEVYE™ for Dry Eye Disease:
.jpg)
In June 2023, the FDA granted approval for Novaliq’s VEVYE™ (cyclosporine ophthalmic solution) 0.1% in treating signs and symptoms of dry eye disease. VEVYE™, previously known as CyclASol®, stands as the first and only cyclosporine solution demonstrating efficacy in alleviating dry eye disease following a 4-week treatment period.
VEVYE™ targets the extensively documented inflammatory root cause of dry eye disease, consistently showcasing early and clinically significant efficacy in addressing both signs and symptoms.
7. Tarsus Pharmaceuticals' XDEMVY™ for Demodex Blepharitis: 
In July 2023, Tarsus Pharmaceuticals obtained FDA approval for XDEMVY™ (lotilaner ophthalmic solution) 0.25% for the treatment of Demodex blepharitis. This marks the first and only FDA-approved treatment specifically addressing Demodex mites, the root cause of the condition.
The approval was grounded in the positive results from two randomized, multicenter, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2), encompassing 833 patients, with 415 of them undergoing treatment with XDEMVY.
Explore XDEMVY, Tarsus Pharmaceuticals' newly FDA-approved treatment for Demodex blepharitis.
8. Regeneron's High-Dose Aflibercept (Eylea HD):
.jpg) In August 2023, Regeneron secured FDA approval for Eylea HD (aflibercept 8 mg), marking a significant development poised to substantially prolong dosing intervals for patients dealing with retinal diseases such as wet age-related macular degeneration (AMD), diabetic macular edema (DME), and diabetic retinopathy (DR).
In August 2023, Regeneron secured FDA approval for Eylea HD (aflibercept 8 mg), marking a significant development poised to substantially prolong dosing intervals for patients dealing with retinal diseases such as wet age-related macular degeneration (AMD), diabetic macular edema (DME), and diabetic retinopathy (DR).
The recommended dosing regimen for Eylea HD is 8 mg (equivalent to 0.07 mL of a solution containing 114.3 mg/mL) every 4 weeks during the initial 3 months for all indications. Following this period, for wet AMD and DME, the dosing frequency is adjusted to 8 mg every 8 to 16 weeks, while for DR, it is recommended to administer 8 mg every 8 to 12 weeks.
Find out about the FDA's approval of Regeneron's high-dose Aflibercept (Eylea HD).
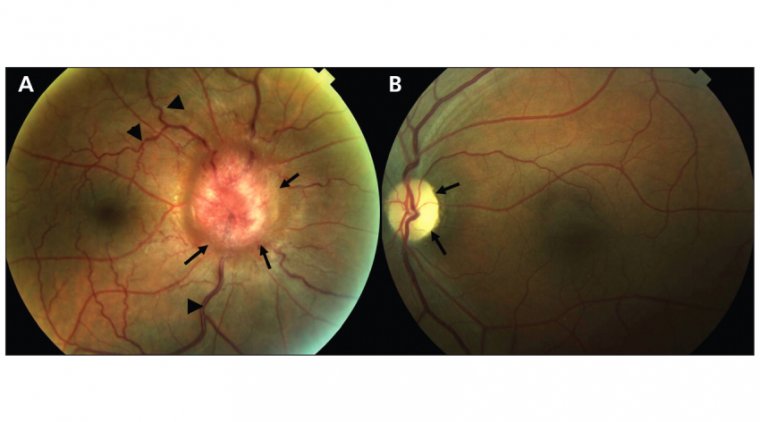
9. Iveric Bio's Izervay for Geographic Atrophy:
.jpg) In August 2023, Iveric Bio received FDA approval for Izervay (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
In August 2023, Iveric Bio received FDA approval for Izervay (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Izervay, a novel complement C5 inhibitor, holds the distinction of being the only FDA-approved treatment for GA. It has demonstrated a statistically significant reduction (P<0.01) in the rate of GA progression at the 12-month primary endpoint in two phase 3 clinical trials.Click here to read the full article.
Learn about the FDA's approval of Iveric Bio's IZERVAY for geographic atrophy treatment.
10. Ocuphire and Viatris' Ryzumvi for Pharmacologically-Induced Mydriasis:
.jpg) In September 2023, Ocuphire Pharma and Viatris jointly announced the FDA approval for Ryzumvi (phentolamine ophthalmic solution) 0.75%. This approval specifically allows its use in addressing pharmacologically-induced mydriasis caused by adrenergic agonists (such as phenylephrine) or parasympatholytic agents (like tropicamide).
In September 2023, Ocuphire Pharma and Viatris jointly announced the FDA approval for Ryzumvi (phentolamine ophthalmic solution) 0.75%. This approval specifically allows its use in addressing pharmacologically-induced mydriasis caused by adrenergic agonists (such as phenylephrine) or parasympatholytic agents (like tropicamide).
RYZUMVI is an anti-microbial preservative-free topical eye drop formulation, featuring phentolamine ophthalmic solution 0.75%. Its primary indication is treating pharmacologically-induced mydriasis triggered by adrenergic agonists (e.g., phenylephrine) or parasympatholytic agents (e.g., tropicamide). RYZUMVI is characterized as a relatively non-selective alpha-1 and alpha-2 adrenergic agonist.
Discover Ryzumvi, the latest FDA-approved treatment by Ocuphire Pharma and Viatris.
11. Genentech's Vabysmo for Retinal Vein Occlusion:
.jpg)
In October 2023, the FDA has granted approval for Genentech's Vabysmo (faricimab-svoa) in the treatment of macular edema following retinal vein occlusion (RVO). This approval marks the third recognized use for Vabysmo, joining its existing approvals for wet age-related macular degeneration (AMD) and diabetic macular edema (DME).
Vabysmo works by inhibiting two signaling pathways associated with various vision-threatening retinal conditions. It achieves this by neutralizing angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A).
12. Orasis' Eye Drug Qlōsi for Presbyopia:
(1).jpg) In October 2023, Orasis Pharmaceuticals received FDA approval for Qlosi (pilocarpine hydrochloride ophthalmic solution) 0.4% for the treatment of presbyopia. The anticipated availability of Qlosi in the United States is expected in the first half of 2024.
In October 2023, Orasis Pharmaceuticals received FDA approval for Qlosi (pilocarpine hydrochloride ophthalmic solution) 0.4% for the treatment of presbyopia. The anticipated availability of Qlosi in the United States is expected in the first half of 2024.
Qlosi, a prescription eye drop, can be used either daily or as needed, up to twice per day. It has demonstrated efficacy within 20 minutes after administration and can sustain its effects for up to 8 hours, as observed on day 15. Through pupil modulation, it enhances near visual acuity, creating a "pinhole effect" and broadening the depth of field, thereby enhancing the ability to focus on nearby objects.
Conclusion
the FDA approvals of these top 10 ophthalmic drugs in 2023 signify a transformative era in eye care, offering diverse and advanced treatment options for various eye conditions. These breakthroughs underscore the commitment of pharmaceutical companies to advancing ophthalmic therapies and improving patient outcomes.
(1).jpg)