Global Pharma Healthcare Issues Nationwide Recall of EzriCare Artificial Tears
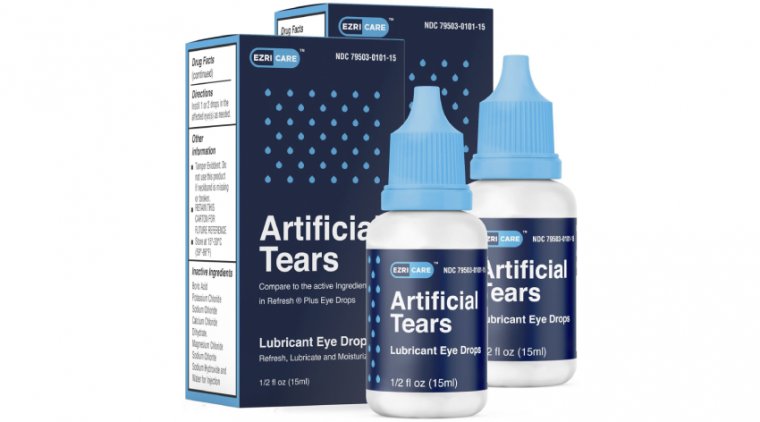
Global Pharma Healthcare is recalling its Artificial Tears Lubricant Eye Drops due to potential contamination. The recall affects all lots within expiry and covers products distributed by EzriCare LLC and Delsam Pharma. This comes after the CDC informed the FDA of a multi-state outbreak of VIM-GES-CRPA infections possibly linked to the use of the artificial tears. There have been 55 reports of adverse events, including eye infections, permanent vision loss, and a death caused by a bloodstream infection.
Artificial Tears Lubricant Eye Drops are used to relieve dryness and discomfort in the eyes caused by minor irritations or exposure to wind and sun. The drops contain 10 mg of carboxymethylcellulose sodium in 1 mL and come in a ½ fl oz (15 mL) bottle with a safety seal. The product is available from EzriCare (NDC 79503-0101-15, UPC 3 79503 10115 7) and Delsam Pharma (NDC 72570-121-15, UPC 72570-0121-15). The eye drops are packaged in a carton box, and are widely available nationwide in the USA through online distribution.
Global Pharma Healthcare is informing its distributors, Aru Pharma Inc. and Delsam Pharma, about the recall and requesting that wholesalers, retailers, and customers discontinue use of the product. The company added that this recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
The FDA has also warned consumers and health care practitioners not to purchase and immediately stop using EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to potential bacterial contamination.
On January 24, 2023, the Centers for Disease Control and Prevention (CDC) recommended immediate discontinuation of the use of EzriCare Artificial Tears, an OTC drop for dry eye, after linking various infections with the drops.
(1).jpg)