Lotilaner Ophthalmic Solution Proven Effective for Treating Demodex Blepharitis
A team of investigators, led by Elizabeth Yeu, MD, from Virginia Eye Consultants in Norfolk, Virginia, reported the safety and efficacy of lotilaner ophthalmic solution, 0.25% (TP-03, Tarsus Pharmaceuticals Inc.), in treating Demodex blepharitis.
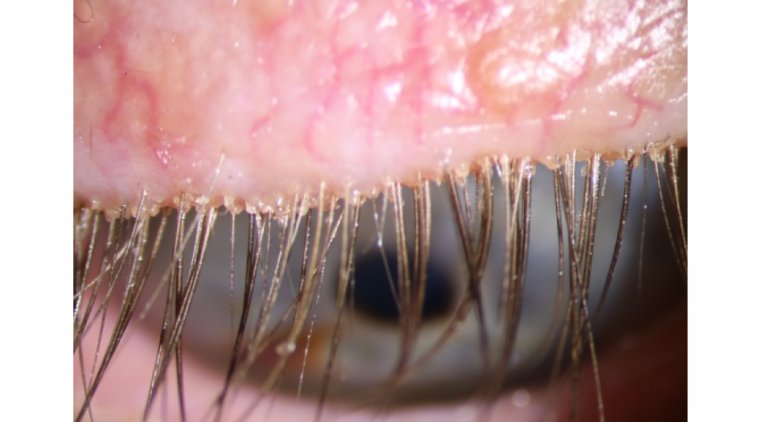
Demodex mites are the most common ectoparasite found on the human skin, and they are associated with dermatologic and ocular diseases, including blepharitis. Dr. Yeu explained that there is currently no FDA-approved treatment for this condition.
The Saturn-1 phase 2b/3 trial was conducted to assess the efficacy and safety of lotilaner ophthalmic solution in treating Demodex blepharitis. The study was a prospective, double-masked, randomized, and controlled clinical trial that included 421 participants.
The patients were divided into two groups, and both eyes of each patient received either the active treatment or vehicle. The treatment was administered twice a day for 43 days, and the participants underwent examination on days 8, 15, 22, and 43 of the study.
In the Saturn-1 phase 2b/3 study, the degree of cure was the primary outcome, which included complete cure, clinically meaningful collarette cure, mite eradication, erythema cure, and composite cure. The researchers also assessed patient comfort with the drops and monitored adverse events. Collarettes, which are cylindrical dandruff, are the hallmark sign of Demodex blepharitis.
Lotilaner Ophthalmic Solution Results
Lotilaner ophthalmic solution showed significant improvement in the treatment of Demodex blepharitis according to the Saturn-1 study. Patients treated with lotilaner showed higher rates of clinically meaningful collarette cure, complete collarette cure, mite eradication, erythema cure, and composite cure compared to the control group. The drops were well-tolerated with 92% of patients rating them as neutral to very comfortable. Mild ocular adverse events were reported, with pain at the instillation site being the most common.
“Lotilaner ophthalmic solution, 0.25%, is the first drug designed to treat and target the underlying cause of Demodexblepharitis,” investigators concluded. “The results of this pivotal study demonstrate that twice-daily use of lotilaner ophthalmic solution, 0.25%, for 43 days is safe and effective for the treatment of Demodex blepharitis compared with the vehicle control. The resolution of Demodex blepharitis, a disease that currently has no FDA-approved treatments, is very promising.”
Reference
Yeu E, Wirta DL, Karpecki P, et al. Lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex Blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2023;42: 435-43; DOI: 10.1097/ICO.0000000000003097
(1).jpg)