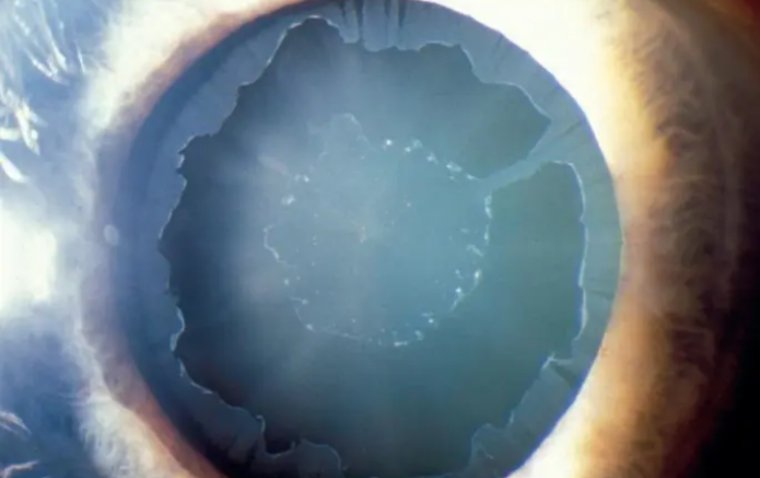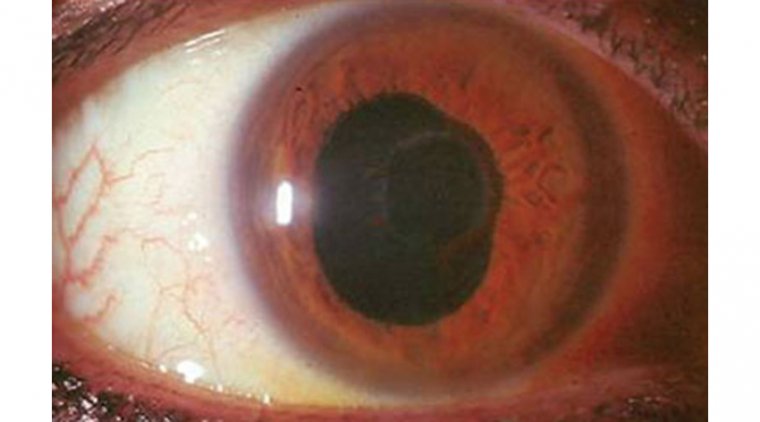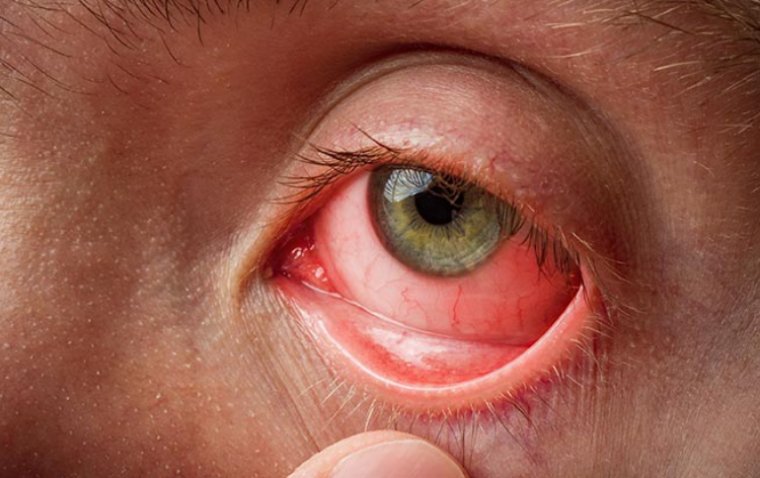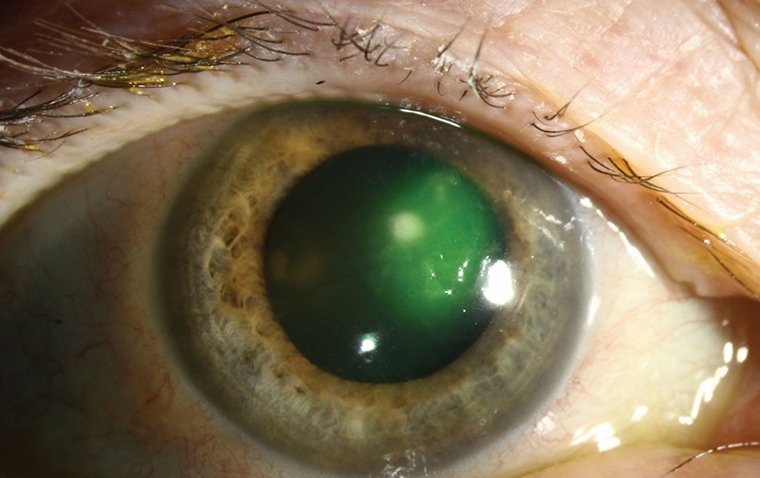
Microbial Keratitis (MK)
Contact lens wearing has been increased globally during recent decades, which is one of the main risk factors for developing microbial keratitis. Microbial keratitis is a severe and dangerous condition that causes cornea inflammation.
Potential Complications of Microbial Keratitis
It can lead to corneal scarring and perforation or even endophthalmitis and visual loss if it remains untreated. Among bacterial, fungal, protozoal, and viral agents which can cause microbial keratitis, bacteria are the most common cause.
What is Endophthalmitis?
Endophthalmitis is an eye condition that causes inflammation and pain inside the eye. It is caused by an infection and can lead to severe vision loss if not treated promptly.
Pathogens and Etiologies of Microbial Keratitis
Microbial keratitis (MK) is a corneal condition that encompasses several different pathogens and etiologies. While contact lens associated MK is most often associated with bacterial infections, other pathogens (fungi, Acanthamoeba species, etc) may be responsible.
Contact Lens Wear and Increased Risk of Complications
Contact lens wear significantly increases the risk of ocular complications, specifically microbial keratitis (MK), which is the most severe complication and is vision threatening. MK is a term that includes bacterial keratitis (BK), fungal keratitis (FK), and Acanthamoeba keratitis (AK).
Bacterial keratitis is an infection of the cornea, the clear outer layer of the eye, caused by bacteria. This condition can cause severe pain, redness, and inflammation, and if left untreated, can lead to vision loss or blindness.
Geographical Differences in Causes of Microbial Keratitis
Geographically, the causes of MK differ. In non-Westernized countries, trauma is the leading cause of MK, whereas in Westernized countries, contact lens wear is equal to or often exceeds trauma as the most significant cause.
Microbial keratitis (MK) is a major cause of corneal scarring and a significant cost burden on the health care system, especially with increasing contact lens use. Additionally, delays in intervention are associated with an increased risk for corneal transplant.
Importance of Immediate Intervention and Treatment
Given this, immediate identification, appropriate treatment, and follow-up care are crucial for successful long-term outcomes. Here, we provide an evidence-based algorithm for managing typical corneal ulcers, most commonly Staphylococcus and Pseudomonas species, effectively.
Acquiring a Thorough Patient History
Acquire a Thorough History Patient’s narration of their disease timeline can provide clues to the infectious organism’s identity and virulence.
Contact lens overuse and poor hygiene are indisputably the most common risk factors for any infectious keratitis; however, we also inquire about previous injury or surgeries, history of ocular surface disease, and exposure to vegetation (is the patient an agricultural worker, gardener, etc.), as these may increase the risk of atypical infections.
Additionally, we determine whether the patient may be immunocompromised or at risk for neurotrophic keratitis (NK). NK may lead to delayed healing, even if the infection is treated appropriately.
We also obtain an accurate outline of the patient’s previous treatments, especially any use of topical corticosteroids. Topical corticosteroids can greatly change the ulcer’s appearance and worsen the pathogenicity of atypical infections.
Identify the Corneal Ulcer - This starts with a systematic evaluation of the entire eye and requires frequent follow-up appointments to assess the virulence of the causative organism. Central lesions are more concerning due to their impact on vision.
Although certainly not a steadfast rule, a rapidly necrotizing stromal suppuration hints more at a bacterial etiology, whereas slower progression can point to atypical causes (i.e., viral, fungal, or protozoa).
Careful measurements of the associated epithelial defect, stromal suppuration, edema, and inflammation can provide helpful information as to the severity and progression of the ulcer.
Classic qualitative associations, such as fungal infections that have feathery edges and satellite lesions or Acanthamoeba with ring infiltrates, warrant further workup, such as additional cultures, stains, or imaging.
The degree of extra-corneal inflammation, such as scleral and anterior chamber involvement, should also be noted, as they are markers of severity and a characterization of the infection. Inflammatory eyelid disease should always be considered, and the eyelids should always be carefully examined.
Finally, if the patient presents with bilateral ulcers, a systemic workup for vitamin A deficiency, HIV, or other immunocompromise should be considered when a direct cause, such as contact lens wear, is not apparent.
These workups may be complex, so a primary care physician can typically help with ordering the appropriate blood work.
Determine Appropriate Treatment - To determine the appropriate treatment for MKs, we use the two-risk tier classification system, low risk or high risk, from the American Academy of Ophthalmology (AAO) Bacterial Keratitis Preferred Practice Pattern:
• Low-risk MKs. These are small, peripheral, or paracentral and non-sight-threatening. The AAO practice pattern suggests small as < 2 mm, without significant stromal involvement or melting.
We recommend treating low-risk patients initially and empirically with the fourth-generation moxifloxacin 0.5% manufactured sans preservatives.
Multiple studies have shown that fourth-generation fluoroquinolones, with their broad-spectrum reactivity to both gram-positive and gram-negative organisms, is comparable to fortified antibiotics in efficacy.
Specific speciation depends on location of the practice and may not be important in these low-risk patients.
However, nationally and in a case series of our local county hospital, Staph. genera are becoming more resistant to fluoroquinolones. Thus, it is important to follow up daily, even in low-risk patients, to assess for improvement.
Should no improvement be noted, we prescribe a drug holiday and refer to the high-risk treatment algorithm.
• High-risk MKs. These are large, sight-threatening, necrotizing, or limbal/scleral-involving lesions. Additionally, patients with atypical features, such as feathery borders, immunocompromise, trauma, corticosteroid use, or those who are unresponsive to topical antimicrobials, are considered high risk for these ulcers.
To arrive at the most appropriate treatment, we typically obtain eye stains and cultures first. Stains are vital to our culturing process, as they provide near-immediate identification. We prefer using both gram and acridine orange stains for standard high-risk MKs, because they have high specificity.
Patients with any atypical history (indolent, not improving on antibiotics, previous use of steroids, high-risk work) or features (feathery edges, satellite lesions, ring infiltrates) should be cultured for atypical infection at the initial scraping.
After staining and culturing, we start high-risk patients on empiric therapy of fortified tobramycin 14 mg/mL and vancomycin 10 mg/mL to 25 mg/mL every 30 minutes to q2h alternating, depending on severity.
This specific combination allows for both gram-negative and gram-positive coverage. It is important to follow up on the stains right away, as the process only takes around 15 to 25 minutes to provide a near-immediate identification.
While some offices, such as those in hospitals and teaching facilities, may be able to process these immediately, we recognize that most private offices likely have to send these out, and results may not be available for 24 hours or more.
Culture sensitivities should be followed daily, although most bacterial growth happens within the first 24 to 48 hours.
If cultures were not fruitful and the patient is still not improving on empiric antibiotic treatment, we consider several reasons: The most obvious is patient non-compliance. Another possibility is a polymicrobial or resistant microbial infection not being treated.
The last possibility is that the ulcer is not a bacterial keratitis, but another, possibly atypical, organism not previously considered.
Due to the long-term nature and toxicity of atypical corneal infection treatments, we usually seek a pathognomonic exam, culture, pathology, or polymerase chain reaction (PCR)– based assay verification before committing the patient to antifungals, antivirals, or antiamoebic drugs.
Therefore, we redo and expand the stain, culture, or PCR after a drug holiday. Typically, a 24-hour drug holiday is sufficient. If there is risk of imminent corneal melt and further vision loss, the culture can be performed without a drug holiday.
After a second negative stain and culture, we typically perform a corneal biopsy if enough stromal thickness exists to reculture as above, including PCRs.
Confocal microscopy is an option for organism identification; however, it is famously user-dependent and may not be as pathognomonic as molecular methods like PCR.
Steroid use for these patients has been a frequent topic of debate. The safest reason for starting high-risk MK patients on steroids is if there is a culture-proven organism that is responding to current antibacterial therapy, but the keratitis burden is large and center involving.
When considering steroids, contraindications to corticosteroid use must be first considered. Topical corticosteroids have been shown to worsen herpetic simplex epithelial, Acanthamoeba, and fungal keratitis and lead to a delay in correct diagnosis and treatment.
We do not recommend starting them if the current antibiotic treatment is not working, as the MK could be from an atypical organism that worsens with steroids.
In a subanalysis of the Steroids for Corneal Ulcers Trials (SCUT), patients with counting fingers or worse vision and central ulcers from highly virulent organisms, like Pseudomonas, showed improvement in long-term vision with the addition of topical steroids after 48 hours of monotherapy with topical moxifloxacin.
Further subanalysis showed a 1-line visual improvement if steroids were started within 3 days of treatment rather than delayed. However, SCUT was not powered for subanalysis, and these results should be seen as guidelines rather than absolute rules.
Although the SCUT trials showed no differences in long-term outcomes with adjunctive topical corticosteroids, it should be noted that steroids were started in the study after bacterial keratitis was confirmed with culture.
In the Nocardia subset of SCUT cases, corticosteroids significantly worsened scar sizes and healing time. The final intervention for highrisk MKs is a therapeutic full-thickness graft for impending perforations or persistently nonhealing large ulcers. Long-term results for the keratoplasty can be varied in active infections, so we usually utilize it as a last resort.
(1).jpg)