Cornell's 'Eye-on-a-Chip' Sheds Light on Mechanism Behind Steroid-Induced Glaucoma
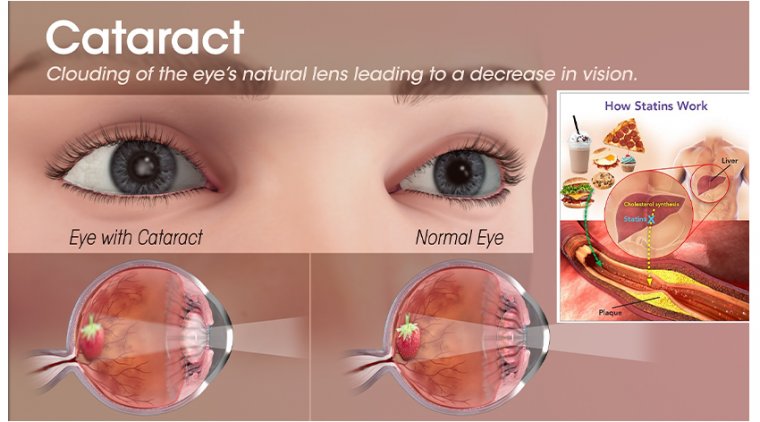
Researchers at Cornell University have identified the signaling mechanism responsible for steroid-induced glaucoma, using a novel 3D “eye-on-a-chip” model that simulates the fluid dynamics of the human eye. The study, published in Nature Cardiovascular Research, presents a significant step toward targeted therapies for a condition currently lacking specific treatment options.
A Microengineered Model to Mimic Ocular Fluid Flow
Led by Renhao Lu, Ph.D. ’24, and senior author Esak (Isaac) Lee, assistant professor of biomedical engineering at Cornell Engineering, the research introduces a microphysiological system (MPS) designed to replicate the anatomical and biophysical complexity of the human eye, particularly the aqueous humor drainage system.
“Steroid-induced glaucoma is a major clinical challenge. There’s no targeted therapy. We just say you are unlucky,” said Esak Lee, Cornell Engineering.
Traditional models using 2D cultures or animal studies have limitations in replicating the human eye’s layered structures and cellular interactions. Lee’s team developed a 3D in-vitro platform incorporating both trabecular meshwork (TM) and Schlemm’s canal (SC) cells, key components in aqueous humor outflow regulation. The device also mimics the curved conduit structure of ocular lymphatic vessels.
Identifying the Steroid-Induced Blockage Mechanism
When treated with the anti-inflammatory steroid dexamethasone, the “eye-on-a-chip” model exhibited impaired aqueous humor drainage, simulating the increased intraocular pressure (IOP) observed in steroid-induced glaucoma.
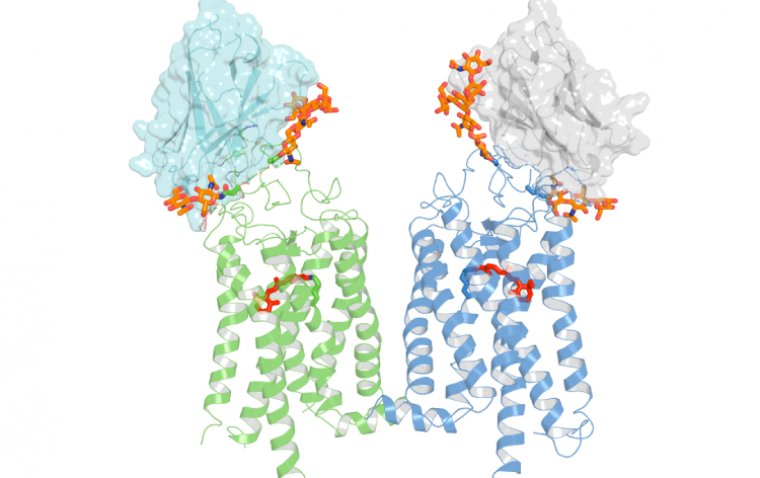
This allowed the team to pinpoint a critical signaling disruption: activation of the ALK5 receptor in TM cells, which led to downregulation of vascular endothelial growth factor C (VEGFC). Under normal conditions, VEGFC facilitates loosening of endothelial junctions in SC cells, allowing fluid to exit into the bloodstream. However, under steroid exposure, the ALK5/VEGFC pathway is disrupted, leading to abnormally tight junctions, increased outflow resistance, and ultimately elevated IOP.
“This communication causes the Schlemm’s canal junction abnormality. The junctions become really thickened or tightened under the steroid, and that junction change increased the resistance of the outflow, causing this glaucoma,” said Esak Lee.
Confirmatory Findings and Therapeutic Implications
The research team validated their findings in a mouse model, confirming that the ALK5/VEGFC pathway plays a causative role in steroid-induced glaucoma. Based on these results, the study proposes two potential therapeutic strategies:
• Inhibiting ALK5 signaling
• Supplementing VEGFC during steroid therapy
These approaches may mitigate the adverse effects of corticosteroids on aqueous humor drainage and prevent IOP elevation.
Advancing Glaucoma Research Through Controlled Genetic Modeling
The microengineered platform offers a versatile tool for studying different glaucoma mechanisms, enabling genetic modifications of TM and SC cells independently, a challenge in conventional animal models.
“It’s complicated and difficult to target one cell type in conventional animal models, but in this system, we could do any genetic modification of these two cell types separately, and then combine them in the device,” Lee explained.
The team aims to explore additional glaucoma-related genetic targets beyond steroid-induced pathways, leveraging the reproducibility and control of their 3D platform to better understand multiple glaucoma subtypes.
Reference:
Renhao Lu et al, Human ocular fluid outflow on-chip reveals trabecular meshwork-mediated Schlemm's canal endothelial dysfunction in steroid-induced glaucoma, Nature Cardiovascular Research (2025). DOI: 10.1038/s44161-025-00704-3
(1).jpg)