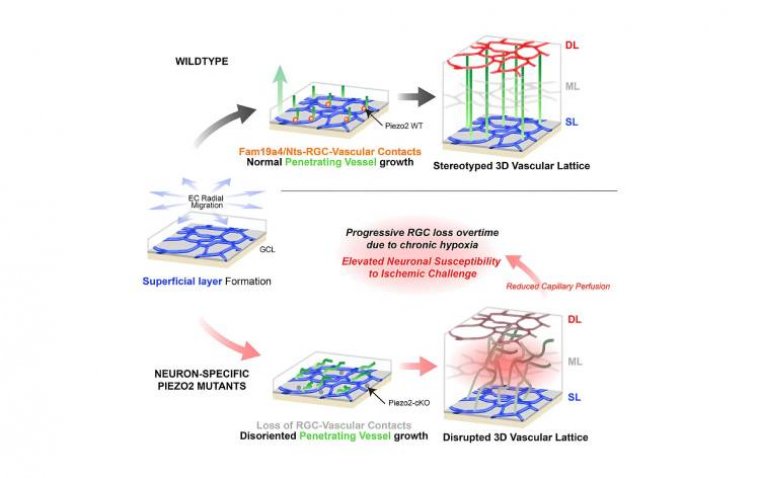
Targeting Cholesterol Metabolism May Offer New Strategy to Prevent Vision Loss in AMD
A new study from Washington University School of Medicine in St. Louis, published in Nature Communications, has identified a potential pathway to slow or prevent age-related macular degeneration (AMD)—a leading cause of vision loss in individuals over 50. The research implicates disrupted cholesterol metabolism in the disease process and suggests that increasing levels of the molecule apolipoprotein M (ApoM) may offer a novel therapeutic approach.
Linking Cholesterol Metabolism to Retinal Degeneration
Using human plasma samples and mouse models of AMD, the research team—led by Rajendra S. Apte, MD, PhD, the Paul A. Cibis Distinguished Professor of Ophthalmology and Visual Sciences—demonstrated that low ApoM levels impair cholesterol processing, contributing to cellular damage in the retina.
This dysfunction mirrors processes seen in cardiovascular disease, supporting a growing body of evidence that connects AMD and heart failure through shared mechanisms of lipid metabolism and inflammation.
ApoM: A Potential Protective Molecule for the Retina
ApoM, known for its anti-inflammatory properties and its role in maintaining cholesterol homeostasis, emerges as a central player in retinal health. The researchers observed that patients with AMD exhibit reduced circulating ApoM compared with healthy controls—a finding consistent with earlier studies showing decreased ApoM in patients with various forms of heart failure.
The team found that ApoM is crucial in enabling the "good cholesterol" pathway to clear harmful lipid accumulations via the liver. In the absence of sufficient ApoM, cholesterol builds up in the retinal pigment epithelial cells, triggering inflammation and degeneration.
Experimental Therapeutic Approach in AMD Models
To test whether elevating ApoM could reverse AMD-like damage, researchers increased ApoM levels in mouse models using genetic engineering and plasma transfer. Results included:
• Improved retinal health
• Enhanced function of photoreceptors
• Reduced cholesterol accumulation
Further investigation revealed that ApoM exerts its effects by activating lysosomal pathways—key mechanisms for cellular waste clearance—and must be bound to sphingosine-1-phosphate (S1P) to be effective.
Clinical and Therapeutic Implications
“Current therapies are limited to late-stage AMD and do not reverse the disease,” said Apte. “Our findings suggest that increasing ApoM could treat or prevent AMD and preserve vision as people age.”
These findings also have implications for cardiovascular disease, where similar mechanisms may be at play. “Both retinal and heart muscle cells are sensitive to low ApoM,” added Ali Javaheri, MD, PhD, co-senior author and assistant professor of medicine at WashU. “By exploring strategies to raise ApoM, we may be able to protect both the eye and the heart from cholesterol-driven damage.”
Toward New Therapies: Industry Collaboration
In 2022, Apte and Javaheri co-founded Mobius Scientific, a Washington University startup focused on developing ApoM-targeted therapies. With support from the Office of Technology Management (OTM), the company aims to translate these findings into novel interventions for AMD and cardiovascular disease.
The researchers plan to further explore ApoM-S1P interactions and their role in regulating lipid metabolism across aging-related diseases, potentially opening new avenues for prevention and treatment.
Reference:
Lee TJ, et al. Apolipoprotein M attenuates age-related macular degeneration phenotypes via sphingosine-1-phosphate signaling and lysosomal lipid catabolism, Nature Communications (2025). DOI: 10.1038/s41467-025-60830-1
(1).jpg)