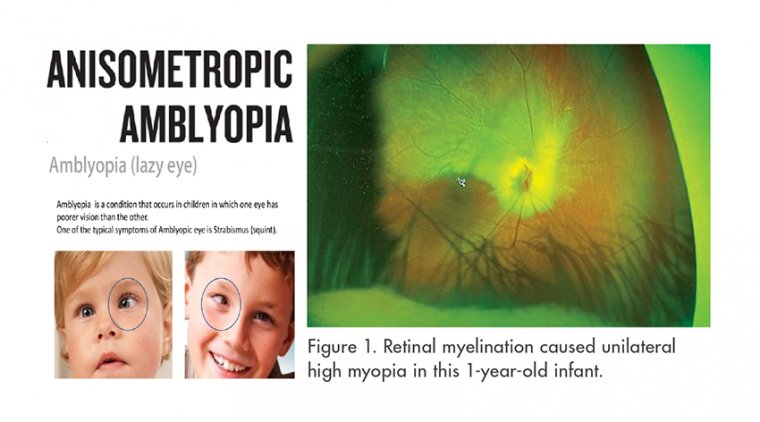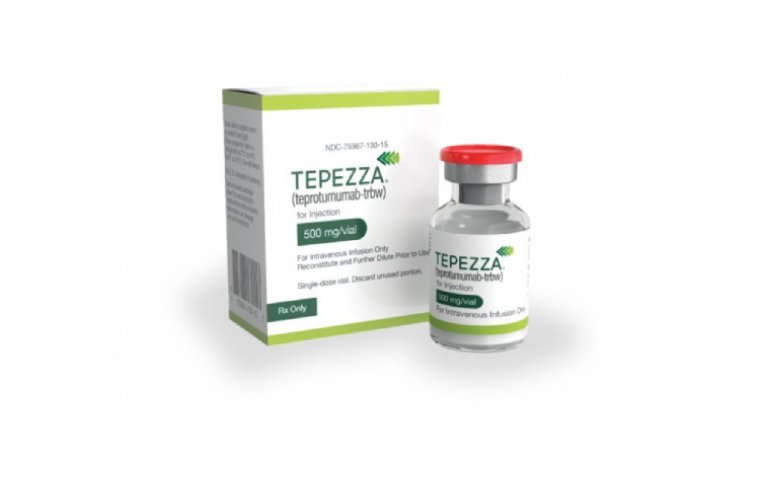
Hearing Loss is Rare in Tepezza Treatment for TED, New Study Finds
A recent study, led by Dr. Raymond Douglas, MD, PhD, revealed that long-term hearing loss is a rare occurrence in individuals with normal audiometry at baseline following treatment with Tepezza [teprotumumab].
Published in the current issue of the prestigious journal Thyroid, the study focuses on the potential otologic symptoms or hearing dysfunction associated with Tepezza treatment, a novel monoclonal inhibitor of the insulin-like growth factor-1 receptor (IGF-1R) approved for thyroid eye disease (TED).
The investigation, involving 52 patients, indicated that those with normal baseline audiometry generally maintained their hearing, with any effects or losses observed primarily in patients with preexisting hearing issues. Audiometry testing was conducted at various intervals, and an independent audiologist evaluated mean thresholds at each frequency per visit, confirming that hearing losses in normal patients are highly unlikely and very rare.
Dr. Raymond Douglas, the lead investigator and author, stated, “Our study conclusively confirmed that hearing loss with Tepezza treatment is, in fact, very rare.” He emphasized the importance of understanding potential adverse effects before administering such drugs and highlighted the necessity of expert patient care and ongoing monitoring for long-term success.
Dr. Douglas, also the founder of Thrive Health IV, emphasized their comprehensive care approach for TED patients, managing Tepezza infusion protocols and conducting proactive audiology testing.
The study's publication coincided with an FDA label warning update for Tepezza, addressing the potential for hearing loss. Dr. Douglas reassured that while monitoring is crucial, the majority of treated patients will not be affected. Tepezza's success rates, comparable to surgical outcomes, make it a viable option, offering relief for TED patients without the need for surgical intervention.
(1).jpg)