General Stages of AMD & Management
Age-related macular degeneration (AMD) is diagnosed by a comprehensive eye exam and other testing.
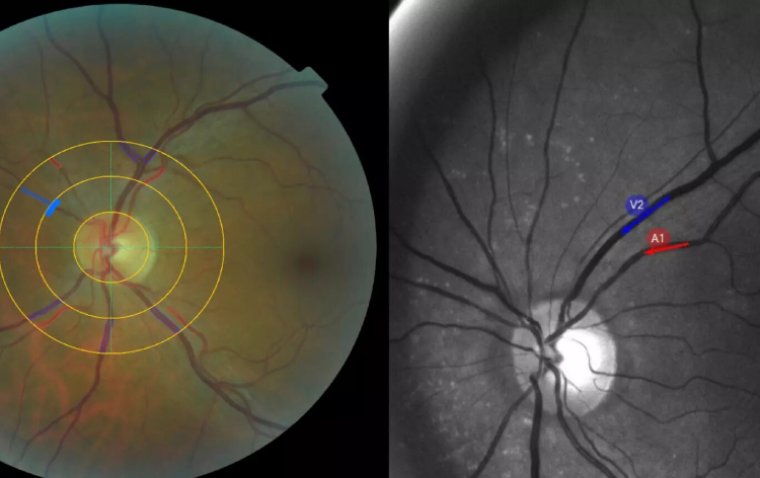
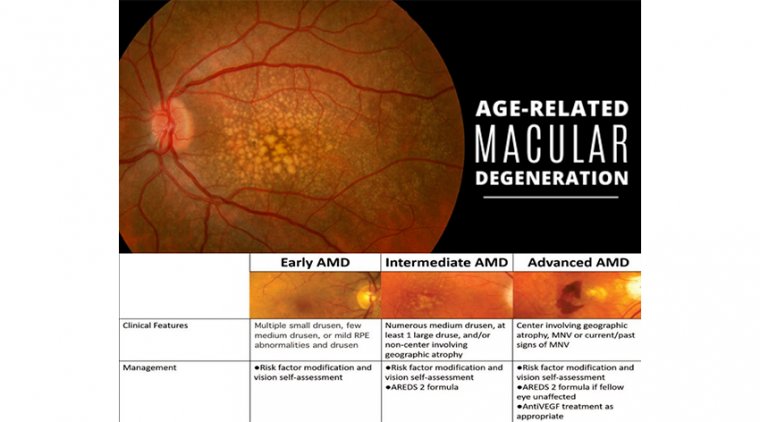
There are three general stages of AMD, partly based on the size and amount of drusen found under the retina on examination.
Early AMD
In early AMD, there is usually no vision loss, and there are small or few medium-sized drusen, which are about the thickness of a human hair. With early AMD, there is a low risk of progressing to advanced AMD within the next 5 years.
Even after 10 years, one study found that only 15% of people with no drusen or small drusen at diagnosis went on to develop large drusen.
Age-related macular degeneration (AMD) is a prototypical chronic ocular condition: It affects millions of people, there is no cure, risk factor modification is a mainstay of treatment, and despite our best efforts, a subset of patients will still progress to significant vision loss.
Intermediate AMD - This diagnosis is important to recognize because individuals with intermediate AMD are at significant risk for developing advanced AMD. In intermediate AMD, there may be some or no vision loss.
In this stage, there are either multiple medium-sized drusen, or at least one large drusen, in one or both eyes, along with changes in the retinal pigment epithelium (RPE), which are cells underneath the retina that support the health of the retina.
AMD is so prevalent that it is often overlooked during the course of a comprehensive eye exam. In fact, though it is estimated that 2.95 million people have AMD in the US alone, a recent study revealed that 25% of AMD patients go undiagnosed.
As eyecare providers, it is our duty to properly identify AMD patients, and then tailor our treatment approach toward the individual patient.
Identification and Classification
AMD is a retinal disease, most often occurring in patients over 50 years of age, characterized by drusen, pigmentary abnormalities, geographic atrophy, and/or choroidal neovascularization.
For the purposes of investigation, the Age-Related Eye Disease Study (AREDS) created a clinical system to classify the various subtypes of AMD.
For daily clinical care, a simplified AREDS classification is used to stage patients within a continuous spectrum. Patients with no or few small drusen are not considered to have AMD. These changes are fairly common and thought to represent age-related changes.
Once patients develop multiple small drusen (125μm), and/ or non-center involving geographic atrophy. Depending on the extent of drusen formation and RPE abnormalities, patients may develop mild visual changes, such as metamorphopsia or reduced visual acuity.
These are the patients for whom proper intervention can have a tremendous effect on preserving vision. As with early AMD, patients should be counseled on risk factor modification and self-vision assessment.
The AREDS2 formula, an oral supplement proven to decrease the rate of conversion to neovascular AMD, should be recommended. Due to the relatively high risk of conversion to advanced AMD, patients should be monitored every 4 to 6 months and self vision assessment should be emphasized.
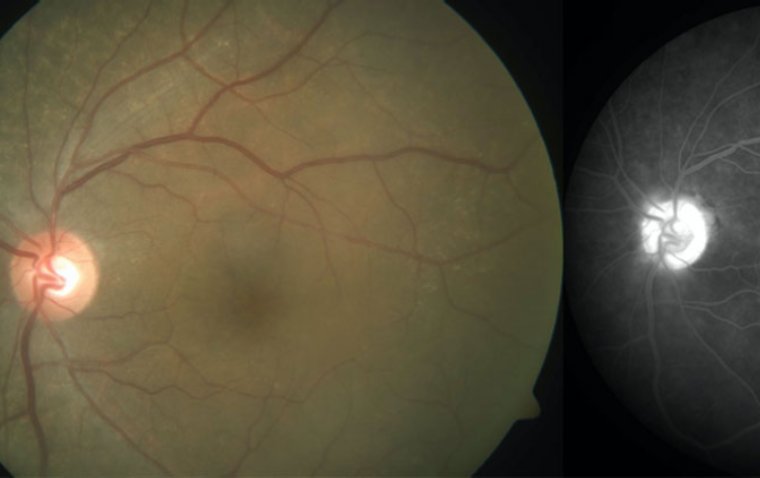
Advanced AMD includes any patient who develops choroidal neovascularization and/or center-involving geographic atrophy.
These are the patients at the highest risk of significant vision loss. Unfortunately, though there are a few potential treatments in the pipeline for geographic atrophy, nothing is currently available.
Contrary to the bleak prognosis of geographic atrophy, neovascular advanced AMD has many available treatments, with even more in development.
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy has become the gold standard for neovascular advanced AMD, and has saved countless patients from significant vision loss.
Despite the almost miraculous effects of intravitreal anti-VEGF therapy, patients are still losing vision to neovascular advanced AMD. The reason for vision loss is multifactorial, but late detection is a major contributor, and one that we as eyecare practitioners can improve.
Real-world data has shown that the average visual acuity upon initiation of neovascular AMD therapy is 20/83, but with newer monitoring technologies, this is something that we can improve.
Focusing on Early Detection
As with any disease, outcomes in neovascular AMD are improved with earlier detection. Studies have demonstrated that patients with better initial acuity when treatment is initiated tend to have a better final acuity.
Intuitively, this makes sense because larger neovascular lesions produce greater hemorrhaging, scarring, and atrophy.
Considering that the average patient may have neovascular AMD for up to a year before treatment initiation, it is no surprise that intermediate AMD patients will lose 3 to 5 lines of visual acuity before their neovascular AMD diagnosis.
Though this data is terrifying, it also shows us that there is a great opportunity to improve management and preserve vision in the 10% to 15% of AMD patients who develop neovascularization.
Classic standard of care tries to detect the conversion to neovascular AMD with semi-frequent in-office visits and home vision assessment, often with an Amsler grid.
An ancillary study to AREDS2, called the HOME study, investigated whether an improvement on the detection of conversion could be made using a remote monitoring program called ForeseeHome.
Results showed that patients using ForeseeHome had excellent outcomes, with 91% of the patients having 20/40 or better vision at the initiation of anti-VEGF treatment.
A recent retrospective data analysis showed that real-world performance resembled the pivotal trial results with 81% of patients maintaining 20/40 or better vision.
(1).jpg)