DRY EYE & OCULAR ALLERGY
Ocular allergy is a common clinical disorder that includes dry eye syndrome in its differential diagnosis.
While ocular allergy treatments have continued to evolve since the early 1990s when the new prescription topical agents became available, there have been no major advances in the treatment of dry eye syndrome other than changes in the chemical structures of various artificial tear formulations.
When allergen identification and avoidance and nonpharmacological treatments give way to drug options, specialists can choose from a variety of topical and oral agents, including antihistamines, mast cell stabilizers, dual-action agents, corticosteroids, and immunomodulators.
Older oral antihistamines are being reformulated into topical drops, new dual-acting agents are being explored, companies are rethinking the role of preservatives, and brand-new categories are making a splash.
Allergic conjunctivitis is reported to affect about 40% of the population, and its prevalence is increasing. Ocular allergies are associated with a significant economic burden as well as quality-of-life concerns.
The global allergic conjunctivitis market was valued at $2.50 billion in 2019 and is predicted to reach $3.05 billion by the end of 2027 on the strength of a 5.4% growth rate. The combination of the condition’s high prevalence, new treatment options, and large investments in clinical trials is driving market growth, according to analysts.
They note that with changing weather and worsening air pollution in some areas, the potential patient population will continue to expand. One trend that has been changing the allergy landscape is the FDA’s Rx-to-over the counter (OTC) switch process.
In 2006, Alcon’s ketotifen product Zaditor became the first allergy drop to switch from prescription to OTC status.
All 3 of that manufacturer’s olopatadine formulations are also available without a prescription: Pataday Once Daily Relief Extra Strength (olopatadine 0.7%, formerly prescribed as Pazeo); Pataday Once Daily Relief (olopatadine 0.2%); and Pataday Twice Daily Relief (olopatadine 0.1%).
“Approval of a wider range of nonprescription drugs has the potential to improve public health by increasing the types of drugs consumers can access and use that would otherwise only be available by prescription,” the FDA said in a statement Novel research is under way, and at the same time, manufacturers are seeking to reformulate available drugs.
Because the eye’s anatomy presents physiological limitations on drug bioavailability, there is much interest in new drug delivery technology. Here is a review of current drug categories and a selection of agents in the pipeline.
Antihistamines
Antihistamines target a key factor in allergic conjunctivitis and have therefore been widely available on the market. Systemic antihistamines historically used for ocular allergy control have different affinities to various antihistamine receptors.
H1 are largely responsible for pruritus, whereas H2 leads to vasodilation. Efficacious antihistaminic treatments continue to be developed; the comfort of topical drops is a key concern. Some patients require the concomitant use of lubricating eye drops and oral antihistamines to maximize control of ocular allergy symptoms.
BILASTINE
Approved for the treatment of allergic rhinitis and urticaria in 2011, bilastine is undergoing allergic conjunctivitis clinical trials as a drop (NCT03479307). The ophthalmic solution 0.6% is being compared with ketotifen ophthalmic solution 0.025% for the treatment of the signs and symptoms of allergic conjunctivitis.
As a second-generation, selective H1-antihistamine, bilastine avoids the sedative effects of first-generation agents.
CETIRIZINE
Another antihistamine, CETIRIZINE (Zerviate, Eyevance Pharmaceuticals) was approved in the United States for topical ophthalmic use. Cetirizine has been used for many years as the oral OTC drug Zyrtec.
Clinical trials of the prescription drop showed significantly decreased ocular itch with a rapid onset of action lasting for 8 hours in moderate and severe patients.
Mast Cell Stabilizers
Mast cell stabilizers include drugs like cromolyn or nedocromil; however, they are rarely used as monotherapy. This class prevents degranulation of mast cells, the release of preformed inflammatory mediators, and the synthesis of additional inflammatory mediators.
These agents block both early and late phases of the ocular surface’s allergic response, reducing hyperemia, itching, and irritation, with differing efficacy among the various agents.
Mast cell stabilizers are most effective when administered prophylactically, requiring a several week loading period for optimal benefit which decreases patient compliance.
They are generally safe and have minimal ocular adverse effects, although they can cause transient burning or stinging on application.
Dual-Acting Agents Combination
Mast cell inhibitor, antihistamine agents work via inhibition of mast cell degranulation and competitive binding of the H1 receptor to block histamine binding. Other cytokines that amplify the inflammatory cascade are also released by mast cells.
Dual-acting agents usually work quickly, typically within 30 minutes of instillation, have better compliance compared with mast cell-stabilizing agents, and generally provide relief of the itching associated with ocular allergy.
Combination products are the most commonly prescribed drugs as first-line treatment; side effects are generally mild.
OTC AND R X AVAILABLE OLOPATADINE—originally a prescription drop and now OTC—was the first dual-acting eye drop to be FDA approved, and thus considered the “gold standard.”
Now it has been joined by prescription agents azelastine, bepotastine (Bepreve, Bausch + Lomb), epinastine, and alcaftadine (Lastacaft, Allergan).
Alcaftadine differs from olopatadine and bepotastine as it possesses an affinity 10 times greater to both H1 and H2 receptors. As mentioned earlier, ZADITOR (ketotifen, Alcon) was the first dual-acting drop to be switched from prescription to OTC status.
Bausch + Lomb received approval for its non-preserved ketotifen formulation, Alaway (OTC), representing the first dual-acting agent approved in the United States as a non-preserved formulation.
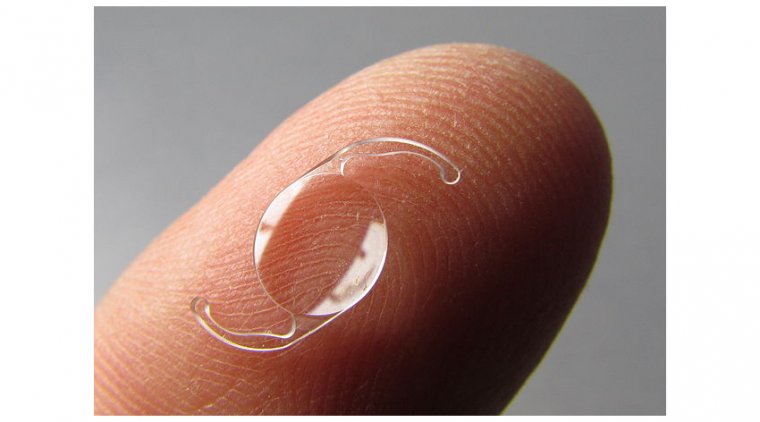
UNDER INVESTIGATION DRUGS DELIVERED VIA CONTACT LENS
It was reported on a study that of 244 patients evaluating the efficacy and safety of a Johnson & Johnson Vision product consisting of the OTC antihistamine/mast cell inhibitor ketotifen delivered via a contact lens for ocular allergy.
Results using the Ora-CAC® Conjunctival Allergen Challenge Model were comparable to direct topical drug delivery and suggest that the lens/ketotifen combination can provide a means of simultaneous vision correction and treatment for lens wearers with ocular allergies, the investigators wrote.
Contact lenses containing epinastine and olopatadine are being studied as well. NSAIDs Topical nonsteroidal anti-inflammatory drugs (NSAIDs) improve ocular itching and reduce mucus secretion, cellular infiltration, erythema, and chemosis.
They work by inhibiting the cyclooxygenase pathway, and thus blocking prostaglandin production. Ketorolac was the first to be approved for quieting the ocular itch associated with allergy; however, it may be associated with discomfort on instillation and is indicated for use for no longer than 2 weeks.
NSAIDs have been associated with cases of corneal melt and are not typically used in clinical practice for allergic conjunctivitis as other drugs remain better options.
Drugs which inhibit phosphodiesterase IV are being investigated for their effects on suppressing activation of the inflammatory cascade. Three PDE4 inhibitor drugs currently approved for the treatment of skin or lung diseases are apremilast, crisaborole, and roflumilast.
Reproxalap is a novel small-molecule immune-modulating covalent inhibitor of reactive aldehyde species (RASP), which are elevated in ocular and systemic inflammatory disease.
Aldeyra Therapeutics has been studying 0.25% reproxalap ophthalmic solution in phase III clinical trials for the treatment of dry eye disease and allergic conjunctivitis.
The company reported statistically significant and clinically relevant improvements in subject-reported ocular itching and tearing and investigator-assessed ocular redness.
Corticosteroids Topical corticosteroids provide the most robust relief from allergic conjunctivitis, effectively relieving a broad range of signs and symptoms.
Patients who report excessive and persistent signs and symptoms that severely interfere with quality of life can be treated in “bursts.”
Ophthalmic steroids should be used judiciously along with steroid-sparing dualactivity agents, and patients must be monitored for well-known steroid-related side-effects such as elevated IOP and cataract development.
BETTER STEROIDS
Newer, safer and C-20 ester-based corticosteroids appear to have an improved IOP side effect profile. Loteprednol 0.02% (Alrex; Bausch + Lomb) is indicated for the treatment of seasonal allergic conjunctivitis with only 1% of patients demonstrating an IOP rise of at least 10 mm Hg.1
UNDER INVESTIGATION DRUG DELIVERED VIA INTRACANALICULAR INSERT
Ocular Therapeutix has reported topline results from its phase 3 clinical trial to evaluate the safety and efficacy of Dextenza intracanalicular insert (dexamethasone 0.4 mg) for the treatment of ocular itching associated with allergic conjunctivitis.
The potential “hands-free” therapy would be administered in the office setting. The bioresorbable insert is designed to release corticosteroid to the ocular surface for up to 30 days. Dextenza met all pre-specified primary endpoints as demonstrated by a statistically significant mean change in ocular itching from baseline, on a subject-reported 5-point scale, at three time points on day 8, the company stated.
IMMUNOMODULATING AGENTS
Topical immunomodulators are currently used in severe allergic ocular conditions, such as vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC). The two most well studied and commonly used are cyclosporine A and tacrolimus, available only as compounded agents.
They work by inhibiting T-cell activation. Other immunomodulatory agents are being investigated; however, use is limited by their low water solubility and lipophilic nature.
Immunobiological modulation, to date, has centered on managing asthma and atopic dermatitis. Omalizumab (Xolair; Genentech), not approved for ocular allergy, may show promise for treatment resistant VKC and AKC.
An IL-4 and IL-13 pathway inhibitor, dupilumab (Dupixent; Regeneron), is being looked at for the treatment of signs and symptoms of AKC in a multicenter, double-masked, randomized, placebo-controlled, parallelgroup, efficacy, safety, and tolerability study (NCT04296864).
The ideal allergic conjunctivitis topical agent is a comfortable drop that has a rapid onset of action, is effective, and long lasting. It is also important that there is not a rebound effect like that seen with long-term use of vasoconstrictors.
Cost considerations will always come into play, although this issue may be shifting as more and more agents move from prescription to over the counter. While there are many competing first-line, dual-acting agents in this category, the reality is that most eye care specialists stick to 2 or 3 agents in clinical practice that they feel work the best in their hands.
Within the dual-acting agent class, we do not believe that there is a significant benefit to using a prescription product versus an OTC agent, when the former’s superiority has not been proven in the clinic.
We do not think eye care specialists can easily justify using a prescription agent if it costs more than an OTC option. If patients fail to get relief with an OTC combination drop, then we consider alternatives.
For patients with recalcitrant ocular allergy or other ocular surface disease that is thought to be in part related to preservatives, we favor a preservative-free compounded version of 0.01% dexamethasone that we have been using for more than 10 years.
Our colleagues have reported its safety and efficacy. Steroids, in our opinion, are really underutilized in chronic ocular surface disease. The proper use of these agents, particularly low dose and unpreserved, can make a huge difference for our patients.
Although undertreated ocular allergy does not usually pose serious consequences for the patient, it does lead to poorer quality of life and decreased satisfaction with outcomes. More treatment options have become available for the acute management of ocular allergic symptoms with many agents moving from prescription to OTC and a number more being looked at in clinical trials.
Although most patients with allergic conjunctivitis will find reasonable relief using currently available topical agents; a segment of this population remains unsatisfied. Therefore, there is a need for the development of safer, more effective anti-inflammatories.
(1).jpg)