Autoimmune Retinopathy: Insights into a Rare Eye Disorder
What is Autoimmune Retinopathy (AIR)?
Autoimmune retinopathy (AIR) is a rare group of diseases characterized by retinal degeneration due to autoimmune mechanisms. There are two primary types of AIR: paraneoplastic retinopathy, associated with an underlying malignancy, and non-paraneoplastic autoimmune retinopathy (npAIR), which occurs independently of cancer.
AIR typically involves bilateral, rapidly progressive vision loss, visual field defects, and photoreceptor dysfunction. The condition is thought to be caused by circulating autoantibodies against the retina, leading to destruction of photoreceptors and degenerative changes in the retina and retinal pigment epithelium (RPE).
What Causes Autoimmune Retinopathy?
The exact causes and mechanisms underlying autoimmune retinopathy (AIR) are not fully understood, but it is believed to be primarily driven by immune system dysfunction, specifically the formation of autoantibodies that mistakenly target proteins in the retina. These autoantibodies lead to the destruction of photoreceptors and widespread degenerative changes in both the retina and the retinal pigment epithelium (RPE).
The RPE, being a crucial component of the blood-retinal barrier and a contributor to immune regulation, often becomes a target in this degenerative process. Several anti-retinal antibodies have been identified in association with non-paraneoplastic autoimmune retinopathy (npAIR). The most significant of these include anti-recoverin, anti-carbonic anhydrase II, anti-α-enolase, and anti-rod transducin-α antibodies.
A possible trigger for the autoimmune response could be a bacterial or viral infection, which might cause a cross-reaction between retinal proteins and bacterial or viral proteins. The resulting retinal damage, affecting photoreceptors, ganglion cells, and bipolar cells, occurs through mechanisms such as caspases and intracellular calcium influx, leading to apoptosis, or programmed cell death. This autoimmune response and the consequent destruction of retinal cells underline the role of autoimmunity in causing retinal damage in AIR.
Symptoms and Diagnostic Process
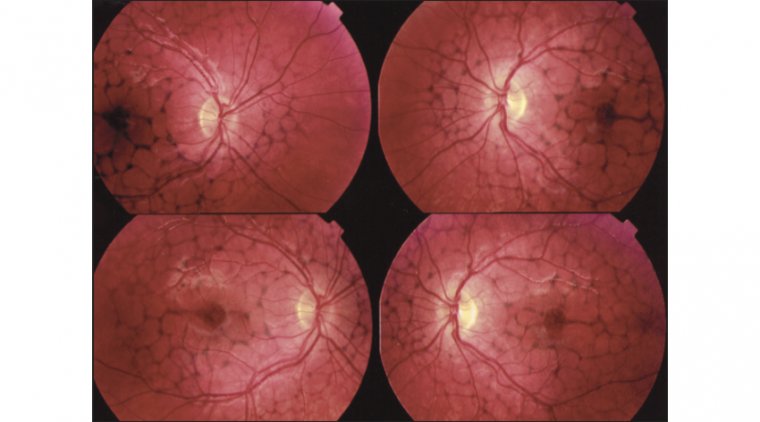
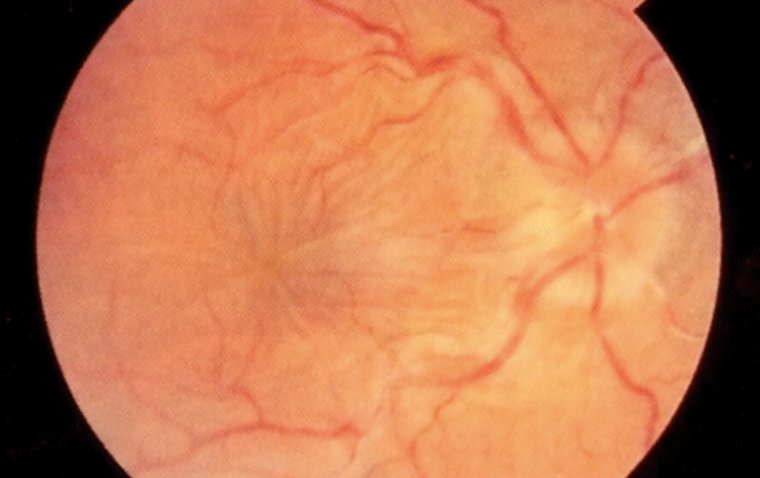
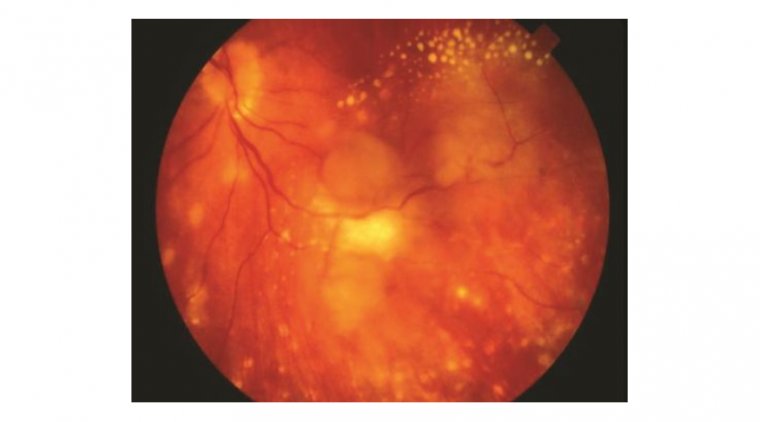
Autoimmune retinopathy presents with a range of symptoms, the most common being subacute vision loss, peripheral visual field loss, flashing lights (photopsias), and night blindness. In early stages, clinical examination may show no significant intraocular inflammation, making the diagnosis challenging. As AIR progresses, symptoms can include narrowing of the retinal vasculature, abnormalities in the retinal pigment epithelium, optic nerve pallor, and mild vitreous cells. The condition is typically bilateral, though it can manifest asymmetrically, and visual acuity is usually preserved until the disease reaches a more advanced stage.
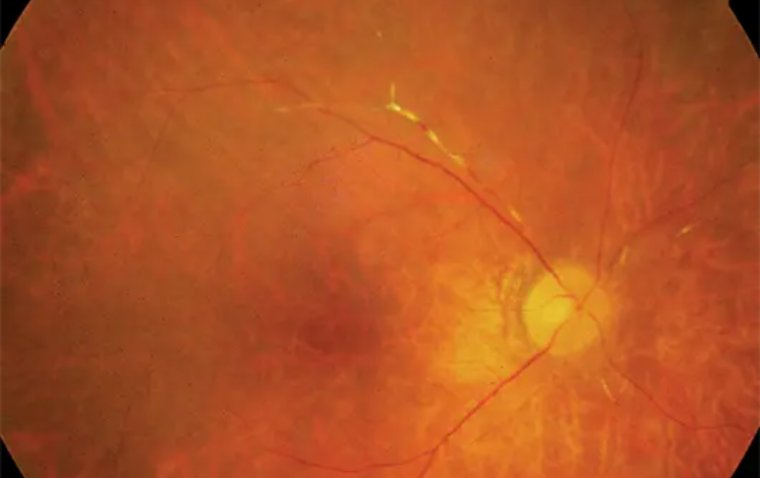
Credit: International Journal of Retina and Vitreous
Diagnosing AIR involves a comprehensive approach due to its nonspecific signs and symptoms, which overlap with many other retinal conditions. The diagnostic process includes:
● A detailed history and clinical examination.
● Visual field testing to assess peripheral and central vision.
● Multimodal imaging, including optical coherence tomography (OCT), which is particularly useful in AIR.
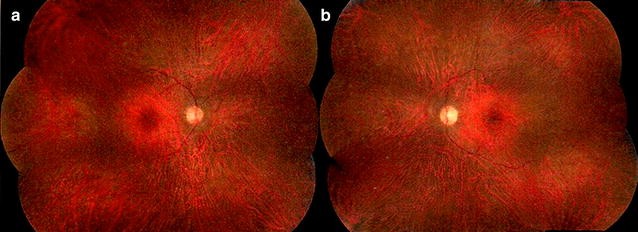
● Fundus autofluorescence (FAF) to monitor disease progression and response to treatment. FAF can reveal diffuse or stippled hyper-autofluorescent patterns throughout the posterior pole and, in some cases, a characteristic parafoveal ring of abnormal hyperautofluorescence.
● Fluorescein angiography, which typically shows no leakage but is performed to rule out other etiologies.
● Full-field electroretinography (ffERG) to evaluate retinal function.
● Retinal antibody testing to identify specific autoantibodies that might be targeting retinal proteins.
● Malignancy evaluation, particularly in cases of paraneoplastic AIR.
This multi-faceted diagnostic approach is crucial in correctly identifying AIR and differentiating it from other retinal conditions with similar presentations.
How is Autoimmune Retinopathy Treated?
The treatment for autoimmune retinopathy (AIR) typically involves immunosuppressive therapy as the mainstay. This approach can include the use of corticosteroids and immunomodulatory drugs. The specific treatment plan for each patient needs to be individualized, considering the subtype of AIR, the patient's overall health, and the severity of symptoms.
Follow-up testing, which includes assessing visual acuity, color vision, visual field, optical coherence tomography (OCT), and full-field electroretinography (ffERG), is generally conducted at 3-month intervals. However, this interval may be adjusted based on treatment type, patient symptoms, and the course of the condition
What is the Prognosis for Autoimmune Retinopathy?
The prognosis for patients diagnosed with autoimmune retinopathy (AIR) presents a complex and varied picture, reflecting the intricate nature of autoimmune diseases. Unlike more predictable ocular conditions, the course of AIR can vary significantly from one patient to another, making personalized prognosis challenging yet crucial.
One of the key characteristics of AIR is its unpredictable disease progression. For some patients, the condition may exhibit a rapid onset of symptoms and deterioration, while others might experience a more gradual decline in vision. This variability underscores the importance of early and accurate diagnosis, followed by prompt initiation of treatment.
The response to treatment in AIR also varies, with some patients responding well to immunosuppressive therapies, leading to stabilization of the condition or even improvement in vision. However, it's important to note that these treatment responses are not universal. Some patients may not respond as effectively, and in rare cases, the disease may continue to progress despite therapy.
The chronic nature of AIR necessitates a long-term management strategy. Regular monitoring, including frequent ophthalmologic examinations and possible adjustments in therapy, is vital to managing the condition effectively. Ophthalmologists play a crucial role in not only providing treatment but also in educating patients about the importance of ongoing care and vigilance.
Summary
Autoimmune retinopathy is a rare group of diseases characterized by retinal degeneration resulting from autoimmune mechanisms. There are two primary types: paraneoplastic retinopathy, associated with malignancy, and non-paraneoplastic autoimmune retinopathy (npAIR), independent of cancer. AIR involves bilateral, rapidly progressing vision loss and photoreceptor dysfunction, attributed to autoantibodies targeting the retina. The exact causes remain unclear, but immune dysfunction leading to autoantibody formation is a key factor. Possible triggers include bacterial or viral infections, causing a cross-reaction with retinal proteins.
Symptoms include subacute vision loss, peripheral field loss, and night blindness. Diagnosis involves a comprehensive approach, including history, clinical examination, visual testing, imaging, and antibody testing. Treatment typically includes immunosuppressive therapy, with varied responses. Prognosis is unpredictable, emphasizing the importance of early diagnosis and ongoing management for effective care. Regular monitoring and long-term strategies are crucial due to the chronic nature of AIR.
(1).jpg)