Update on Recalled Eye Drops: Bacterial Infection Can Spread Person-to-Person
*This news has been updated, please click the link to see the updated version.
The Centers for Disease Control and Prevention (CDC) have expressed concerns that a drug-resistant bacterial strain, linked to imported eyedrops from India, has spread from person to person in a Connecticut long-term care center, which may lead to it gaining a foothold in the U.S. healthcare settings.
According to infectious disease specialists, the strain is particularly challenging to treat using current antibiotics and has not been previously identified in the U.S. The CDC has traced 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had their eyeballs surgically removed due to infection.
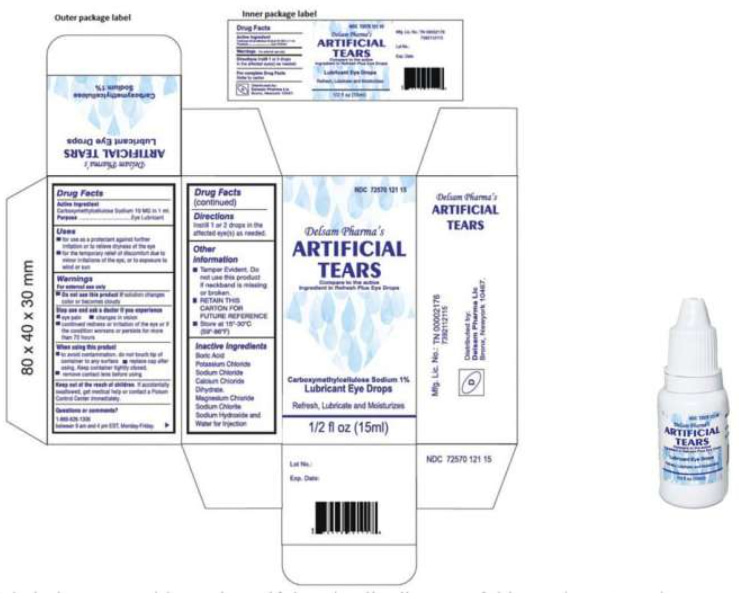
Despite the FDA halting imports of the product, these outbreaks have shed light on the regulatory loopholes surrounding the control of imported medications.
The FDA acknowledged that it had not previously conducted an inspection of the Indian factory that produced the contaminated eyedrops before the infections were reported. However, the agency has since visited the plant, which is managed by Global Pharma Healthcare.
For a long time, the agency has faced criticisms for its negligence in conducting inspections of foreign manufacturing facilities in India and China, which are the largest suppliers of drugs and raw materials used in producing medications. The agency has had to deal with other instances of contaminated foreign-made products, such as blood pressure medicines suspected of containing cancer-causing agents and lethal batches of heparin, resulting in massive recalls.
The FDA has stated that it is working together with the CDC and has requested that retailers ensure the removal of the mentioned products from their shelves.
The latest incident has linked the use of certain eyedrops to a bacteria strain that is more drug-resistant than the bacteria the C.D.C. typically observes, which accounts for around 150 cases each year, usually in intensive-care settings. The CDC's lead investigator for antimicrobial resistance, Maroya Walters, confirmed this.
.jpg)
Dr. Walters has further stated that the emergence of this new strain could change the current situation. At the Connecticut center, where the incident occurred, the bacteria was observed spreading among asymptomatic patients who had the bacteria colonized in their bodies. Spread of the bacteria often occurs when patients come into contact with common items or when healthcare workers transmit the germs.
The bacterium that is associated with the eyedrops, drug-resistant Pseudomonas aeruginosa, is already a major worry for healthcare providers. This is particularly true for individuals with weakened immune systems, nursing home residents, and patients with invasive medical devices such as catheters and breathing tubes.
Dr. David van Duin, who specializes in infectious diseases at the University of North Carolina School of Medicine, noted that resistant pseudomonas is particularly challenging to eliminate. It clings stubbornly to sink drains, water faucets, and other moist environments within healthcare facilities. Moreover, patients who develop bloodstream infections can also be difficult to treat.
Additionally, the FDA has announced a recall of Delsam Pharma's Artificial Eye ointment due to potential contamination. This ointment was produced in the same factory as the affected eyedrops.
Although the FDA mandates a pre-approval inspection of prescription drug manufacturing plants, there is no such requirement for facilities that produce over-the-counter medications like artificial tears. Moreover, the agency's inspection rates have decreased significantly since the onset of the pandemic, exacerbating the issue.
“I think we are going to see the impact of this play out over the course of months to years,” Dr. Walters said.
(1).jpg)