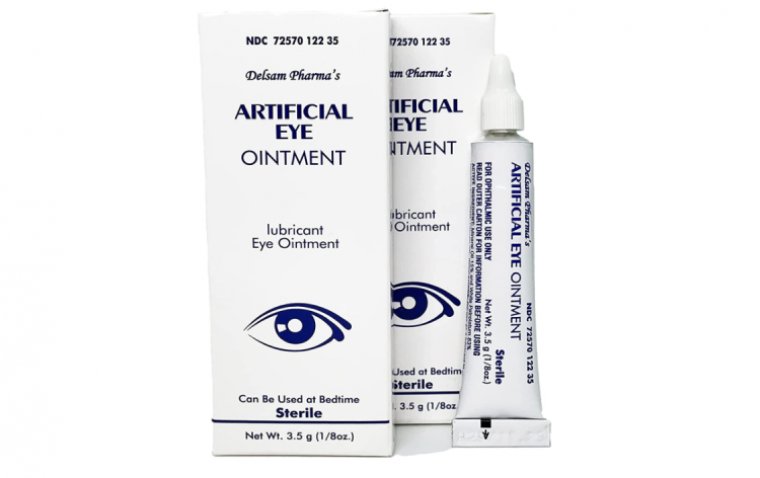
FDA Extends Warning on Eye Care Products to Include Delsam Pharma's Artificial Eye Ointment
The FDA has issued a warning to consumers and healthcare professionals not to purchase or use Delsam Pharma's Artificial Eye Ointment due to potential bacterial contamination. This eye oinment is an over-the-counter product, intended to be sterile and produced by the recalled EzriCare Artificial Tears’ manufacturer, Global Pharma Healthcare Private Limited. The company agreed to initiate a recall.
EzriCare Artificial Tears were recalled last month after there had been reports of a multi-state outbreak of a highly drug-resistant bacteria (Pseudomonas aeruginosa bacteria) that has hospitalized and blinded patients.
The FDA has faulted the company for numerous violations and has prohibited the import of the company's items into the United States, CBS News reported on the matter.
Earlier this year, an outbreak of the rare and extensively drug-resistant Pseudomonas aeruginosa bacteria, which had never been seen in the United States before, caused hospitalizations and blindness in at least 16 patients across 12 states. The U.S. Centers for Disease Control and Prevention issued a nationwide health alert regarding the bacteria.
One patient in Washington state died when the infection spread to the bloodstream, and five others were permanently blinded, while one had to undergo surgical removal of the eyeball. Federal investigators discovered that multiple patients had opened EzriCare eye drops contaminated with the bacteria, although three unopened bottles showed no signs of contamination. The investigators are conducting further tests on additional bottles.
CBS News reported that while most patients purchased the drops online, one reported buying it from a Costco warehouse. Despite the FDA's warning against it, the Delsam Pharma Artificial Eye Ointment remains available for purchase on multiple websites, including Amazon.
(1).jpg)