Presbyopia Correcting Drops
The eye drops don’t treat the cause of presbyopia but help treat its symptoms. Instead of targeting the eyes’ lens, the drops make the eyes’ pupils smaller, creating a pinhole effect that increases the depth of focus.
The eye drops are a pilocarpine solution meant to treat the symptoms of presbyopia, an age-related condition that causes gradual loss of the eyes’ ability to focus on nearby objects.
Presbyopia is caused by the loss of elasticity in the eyes’ lens, which makes it harder to change shape when it needs to shift focus from faraway objects to closer ones.
When it comes to correcting presbyopia, choice is a crucial theme. Patients seek options that match their lifestyle and where they are in their journey through presbyopia. Current and emerging surgery-based presbyopia-correcting strategies are homing in on the sweet spot of fewer trade-offs and more benefits.
This can be seen with newtechnology IOLs and laser-based procedures that provide a more seamless and personalized range of vision with freedom from eyeglasses to suit patients’ lifestyle and how they use their vision.
But where does that leave early presbyopes and even older patients without cataracts? What about the plano pseudophake? Many of these patients are seeking a less invasive and permanent strategy, yet there isn’t much physicians can offer apart from eyeglasses and contact lenses.
That is about to change in a dramatic fashion with presbyopia-correcting drops, which represent a huge opportunity to treat the more than 128 million Americans with presbyopia.
“Incipient presbyopia is the first stage in a continuum of lens dysfunction where patients are just learning about the effects on their quality of life as they begin to need reading glasses or bifocals to function adequately,” says George O. Waring, IV, MD, founder and medical director, Waring Vision Institute, Mount Pleasant, S.C.
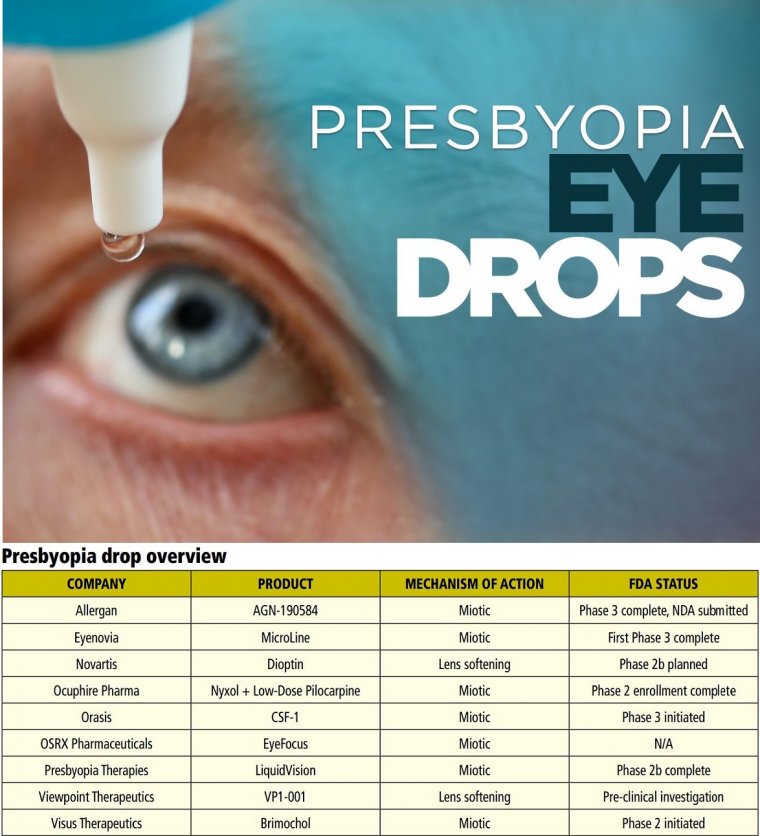
“We are on the doorstep now of having topical drops, of which there are multiple in the developmental pipeline with varying mechanisms of action [MOA], for the treatment of presbyopia.
This is very exciting because it expands our treatment armamentarium on how we can address lens dysfunction across the full spectrum — even at the earliest stages.”
The two main MOAs being investigated for presbyopia-correcting drops are miotics, which leverage the pinhole effect to increase the depth of field, and lens softening, based on the assumption that lens stiffening and loss of flexibility are presbyopia’s main causes.
The ideal agent should recover accommodation and provide good vision at all distances with minimal side effects. Other considerations include the time to onset of action, duration of effect and the use of preservatives.
MIOTICS Abbvie/Allergan AGN-190584
Earlier this year, Allergan submitted a New Drug Application to the FDA for AGN-190584. The submission is based on data from two Phase 3 studies evaluating efficacy, safety and tolerability of its proprietary formulation of pilocarpine 1.25%.
The agent met the primary endpoint reaching statistical significance in improvement of ≥3 lines of near vision in mesopic conditions without a loss of distance vision compared with vehicle.
There were no treatment-emergent serious adverse events (AEs) observed in any AGN190584-treated participants (N = 750), and the most common treatment-related non-serious AEs were headache and conjunctival hyperemia (≥5%).
“The docket has been submitted, and, if approved, this would be the first to market topical therapy for the treatment of presbyopia in the United States,” says Dr. Waring, lead investigator for the drug.
“This MOA for the proprietary formulation of dilute pilocarpine was shown to be safe and efficacious and met the FDA-mandated endpoints.”
Eyenovia MicroLine
The Phase 3 VISION-1 study evaluated the safety and efficacy of Eyenovia’s 1% and 2% pilocarpine Micro-Array Print formulations vs placebo, administered via the company’s proprietary Optejet dispenser.
Recently announced results showed a statistically significant proportion of subjects treated with MicroLine had a three-line or more improvement in distance-corrected near visual acuity (DCNVA) vs placebo in low-light conditions at 2 hours post-treatment.
The company states that MicroLine was very well tolerated, and AEs were all mild in nature with no serious AEs. In a post-study survey, 70% of study participants reported strong interest in using MicroLine for near vision improvement should it be approved.
The Optejet device has many advantages, says David Wirta, MD, a study investigator with the Eye Research Foundation.
“It delivers the solution in a well-distributed mist, so you don’t have to put as much liquid on the surface of the eye to get the same benefits. It is convenient and cleaner, making it easier to use on the go, with less chance of absorption by the body.”
Ocuphire Pharma Nyxol + Low-Dose Pilocarpine
The company has enrolled the first patients of an expected 152 in VEGA-1, its Phase 2 proofof-concept trial to evaluate a combination kit of Nyxol — preservative-free phentolamine 0.75% — plus low-dose pilocarpine in presbyopia.
The primary endpoint is percentage of patients with ≥3 lines improvement of binocular DCNVA. Ocuphire reported that data from multiple Phase 2 trials showed Nyxol alone reduced pupil diameter by approximately 20% and has significantly improved near visual acuity by one line for >24 hours after an evening instillation.
Orasis Pharmaceuticals CSF-1
Based on Phase 2b data, the company has initiated Phase 3 trials NEAR-1 and NEAR-2 to evaluate preservative-free CSF-1, sub-glaucoma dose pilocarpine with a proprietary multi-faceted vehicle, in approximately 600 participants.
The primary endpoint is percentage of patients with ≥3 lines of near VA improvement at 40 cm and no loss in BDCVA ≥5 letters at 4 m. Phase 2b evaluated 166 patients with b.i.d. administration: 47% achieved ≥3 line improvement (P=.0002) vs baseline (16%), and 80% achieved a ≥2 line improvement (P=.0001) vs baseline (43%).
No negative impact on distance and night vision was reported, and the treatmentrelated AEs were mild and temporary. In the studies, there was no diminished effect on distance vision, says Nicole Fram, MD, managing partner at Advanced Vision Care in Los Angeles.
“You can’t take away one problem and give patients another problem.” She adds that the Orasis formulation is preservative free, so it will not add to any preexisting dry eye.
“Another advantage is that it is titratable, so it is in the patient’s control, which is nice. They aren’t ‘locked in’ to an extended duration of effect if they want to go back to using their reading glasses later, for example, or in the event there are some slight side effects.”
OSRX Pharmaceuticals EyeFocus
This compounded miotic eyedrop for the treatment of presbyopia contains low concentrations of pilocarpine, phenylephrine, pheniramine and ketorolac.
According to the company, it will be available in two strengths, labeled as EyeFocus and EyeFocus+. Luis Felipe Vejarano, MD, introduced the presbyopia medication FOV Tears internationally in 2016 and helped the company develop the agent.
It is similar to his formulation, and he expects EyeFocus to be similarly effective. Because this is a compounded product, it will not follow the FDA regulatory pathway. A proof-of-concept study evaluated nine presbyopic patients aged 44 to 64 years over 2 days, and the average DCNVA improvement was three lines.
At hour 1, reading vision was 20/40 in 66% of patients. Reading vision was 20/40 in 89% of patients and 20/20 in 66% to 71% of patients during hours 3 to 5, and 78% of patients maintained 20/40 reading vision for 8 hours.
Presbyopia Therapies PRX-100/Liquid Vision
This agent uses aceclidine, a novel and new chemical entity in the United States, to cause miosis with no measurable induced myopia, which the company notes improves near and intermediate vision without impairing distance vision.
The company’s Phase 2b study showed PRX-100 was associated with a pupil size ranging from 1.5 mm to 2 mm, providing significant uncorrected near vision improvement with no distance vision blurring.
The drug works within 30 minutes and lasts for at least 7 hours. According to the company, aceclidine’s unique MOA allows it to target the full presbyopic market, from ages 45 to 70, with a broader range of refractive error, from -4.50 D to +1.50 D and up to 2.00 D of astigmatism.
The company is expecting to commence its Phase 3 trial as part of its ongoing development plans. “PRX-100’s exclusive and patent-protected active ingredient, aceclidine, distinctively targets the pupil creating a seamless depth of focus without causing a significant anterior shift of the iris-lens diaphragm, which would impact distance vision,” says refractive surgery pioneer Marc Odrich, MD. ”
The rapid onset of action would allow patients to try it in the clinician’s office. PRX-100 eyedrops achieved a pupil size sweet spot and restored near vision for a good part of the day, turning back the clock 10 years or more for the vast majority of presbyopes.”
Dr. Odrich is associate professor at the University of Virginia Department of Ophthalmology, Charlottesville, and a member of Presbyopia Therapies advisory board.
Visus Therapeutics Brimochol
Brimochol is a proprietary combination of carbachol plus brimonidine that reportedly can last up to 8 hours.
The agent has entered Phase 2 trials and is expected to enroll 40 patients to evaluate the safety and efficacy of two formulations of the agent; the primary endpoint is the percentage of subjects gaining ≥3 lines in near visual acuity without losing distance vision.
The company expects to have top-line Phase 2 results at the end of the second quarter of this year and will start two Phase 3 studies expected to enroll more than 500 patients in the third quarter.
Carbachol may have a longer duration of effect compared with pilocarpine, and the company notes that brimonidine appears to mitigate some of the adverse effects of miotics like hyperemia, headache and brow ache.
Previously published data on 57 patients showed that patients treated with the combination achieved a mean 5 lines of near visual acuity improvement out to 12 hours with no complaints of headache or brow ache and no loss of distance vision.
The data on Brimochol is very positive, says Neda Shamie, MD, partner at the Maloney-Shamie Vision Institute in Los Angeles and a member of Visus Therapeutics’ clinical advisory board. “The duration of action was at least 8 hours, which seems to be the sweet spot, and is unique to this formulation, which is a big plus.
The side effect profile in terms of brow ache and headache that we know is common to pilocarpine is far less than what I have seen with similar drops.”
Lens Softening
Novartis UNR844-CL/Dioptin UNR844 (Dioptin) is an ester of naturally occurring R-lipoic acid and choline that penetrates the cornea and is metabolized into choline and dihydrolipoic acid (DHLA).
It is believed that DHLA acts to reduce the disulfide bonds between lens proteins that occur with age, potentially restoring lens elasticity.
A Phase 2 study of 78 subjects, aged 45 to 55 years, did not meet its primary objective as there was no significant difference in mean change in DCNVA between UNR844 and placebo (difference of 1.6 letters, ([90% CI: -1.34, 4.56]; P=0.1832).
In a study abstract, the authors stated that due to high variability in DCNVA measures in both groups (but to a greater extent in the placebo arm, which was heavily tailed with outliers), a post-hoc non-parametric analysis was performed.
The median difference between UNR844 and placebo was four letters (P=0.0924), closer to previous Phase 1/2 study results that showed the agent associated with a 5-letter improvement in DCNVA.
There were no clinically meaningful differences in ocular or systemic side effects between UNR844 and placebo, and the authors noted that further clinical development is planned in a Phase 2b dose-finding study.
“This [MOA] is exciting because it is working at the root of the problem,” says Dr. Fram. “This would be more of a long-lasting change rather than just a miotic change of the iris causing a smaller pupil size.”
Viewpoint Therapeutics VP1-001
The protein alpha-crystallin is an important component of the eye’s natural lens, which is thought to allow it to be transparent and flexible.
With age, these proteins become prone to misfolding, leading to a destabilization of structure that in turn creates clumping.
This causes the lens stiffening that is at the root of presbyopia and light scattering seen in cataract development. Currently in preclinical investigations, the company is looking at the molecule VP1-001 as a way to stabilize alpha-crystallin to treat presbyopia and cataracts.
Surgeons often mention the so-called Holy Grail of presbyopia correction — an option that would restore the accommodative ability of the natural lens in all lighting and across all distances.
Pharmaceuticals may move the industry closer to realizing that dream for many patients. Experts are not saying there will be no need for readers, multifocal contacts or other surgical options. What is likely, however, is that these drops will be used to supplement these other strategies.
(1).jpg)