Novel Treatment Dramatically Slows Down Progression of AMD, RP
A breakthrough study has unveiled a promising advancement in the treatment of incurable eye diseases such as age-related macular degeneration and retinitis pigmentosa.
Researchers have successfully integrated anti-inflammatory drugs into a hydrogel, aiming to combat inflammation in the retina while efficiently delivering medication to the affected area.
Age-related macular degeneration and retinitis pigmentosa pose significant threats to vision, gradually causing blindness by damaging photoreceptor cells in the retina. Age-related macular degeneration, in particular, affects the central part of the retina, known as the macula, and stands as the leading cause of blindness among individuals aged 65 and older. On the other hand, retinitis pigmentosa is a genetic disorder characterized by the progressive death of photoreceptor cells, initially leading to night blindness and eventually resulting in vision loss.
Current treatment options for these conditions include intraocular injections of anti-inflammatory drugs to mitigate retinal damage. However, the effectiveness of these injections is limited by the duration of drug retention in the eye, necessitating frequent clinic visits for re-administration.
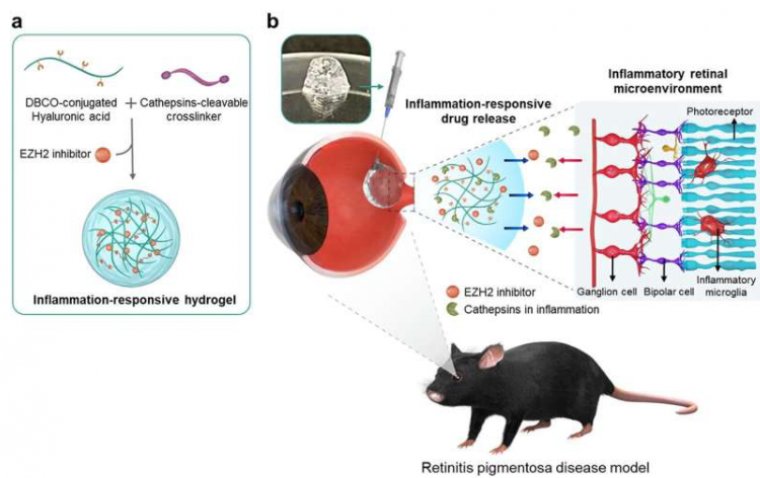
In a pioneering effort, the research team used a substance targeting the inflammatory factor EZH2, which contributes to retinal degeneration, alongside an anti-inflammatory agent.
In mouse models with retinal degeneration, injections of the anti-inflammatory drug significantly slowed the progression of the disease, as detailed in a paper published in the journal npj Regenerative Medicine.
The researchers devised a hydrogel that degrades gradually upon encountering the enzyme cathepsin, prevalent in inflammatory environments, facilitating sustained release of anti-inflammatory drugs. When administered to mice with retinal degeneration, the inflammation-responsive hydrogel led to a remarkable reduction in retinal inflammatory factors by approximately 6.1%. Moreover, the hydrogel demonstrated a fourfold increase in protective effects on photoreceptor cells, crucial for preserving vision.
.jpg)
Overexpression of inflammatory factors and cathepsins in retinitis pigmentosa in vivo model. Credit: npj Regenerative Medicine (2023). DOI: 10.1038/s41536-023-00342-y
Significantly, the hydrogel, formulated with hyaluronic acid, mimics the mechanical and optical properties of the vitreous humor in the eye. This feature enables personalized degradation rates tailored to individual patients, thereby minimizing the need for repeated injections.
The newfound technology bears the potential to alleviate the economic burden and risks associated with outpatient visits for visually impaired patients. By reducing the frequency of hospital visits, especially for those in the early stages of symptoms, the innovation promises to enhance quality of life.
Dr. Maesoon Im of the Brain Science Institute, along with Prof. Seung Ja Oh of Kyung Hee University and Prof. Kangwon Lee of Seoul National University, spearheaded the research endeavor. Looking ahead, Dr. Im expressed intentions to digitize treatment parameters for future commercialization and assess the long-term stability of the drug delivery system. Prof. Oh highlighted the potential applicability of the inflammation-responsive delivery system in other retinal diseases, underscoring the broader impact of this groundbreaking technology.
Reference
Hyerim Kim et al, Effective protection of photoreceptors using an inflammation-responsive hydrogel to attenuate outer retinal degeneration, npj Regenerative Medicine (2023). DOI: 10.1038/s41536-023-00342-y
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
(1).jpg)