Dry Eye Treatment & Cyclosporine
Dry eye disease (DED), also known as keratoconjunctivitis sicca (KCS), is a common disorder of the eye affecting millions of people around the world. The prevalence of DED ranges from 5% to 33% worldwide with increased prevalence among adult women and Asians.
Symptoms of DED include discomfort, pain, burning, foreign body sensation, and visual disturbances. Dry eye negatively affects the quality of life and results in approximately $6 billion in annual costs in the US.
Dry eye is commonly seen alongside many ocular disorders including glaucoma, cataracts, and refractive errors. Pharmacological treatments for ocular disorders often contain preservatives such as benzalkonium chloride.
These preservatives can cause DED signs and symptoms including decreased epithelial cell integrity, increased epithelial cell apoptosis, eye irritation, and increased risk for ocular allergies and delayed hypersensitivity reactions.
Surgical interventions to treat ophthalmic disorders and diseases can also cause dry eye due to the resultant inflammation from the length of time in surgery, incision site disruption to the corneal nerves, and type of post-surgical medication.
Post-trabeculectomy patients have elevated tear film osmolarity and symptoms of dry eye; intraoperative use of lubricating substances can decrease post-surgical dry eye symptoms and treatment with dry eye medication post-operatively can help alleviate surgery-induced dry eye.
Dry eye symptoms following cataract surgery occur in 42%, 15%, and 9% of eyes of patients at 1 week, 1 month, and 3 months after surgery, respectively.
Additionally, new research demonstrates the bilateral impact on the corneal sub-basal nerve complex after cataract surgery, potentially explaining worse dry eye symptoms after cataract surgery on the second eye.
Cyclosporine
Cyclosporine is a neutrally charged hydrophobic molecule with low aqueous solubility, which poses challenges in making a safe and effective ocular drug delivery system.
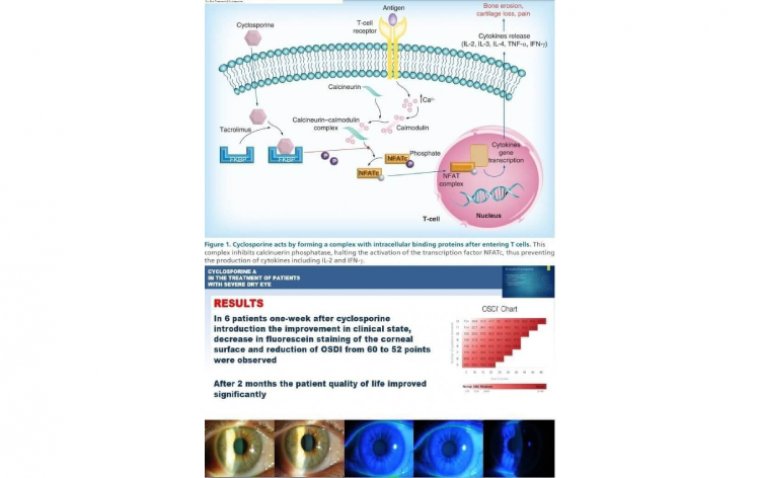
Cyclosporine reduces the underlying inflammation associated with DED that interferes with tear production.
Initially, cyclosporine ophthalmic solutions were formulated in oil-based solvents such as castor oil or corn oil; however, oils caused side effects such as blurred vision, burning, and stinging, and were poorly tolerated.
Additionally, these oils provided low bioavailability of cyclosporine A to the ocular targets. The limitations of oil-based cyclosporine A formulations lead to a need for cyclosporine A formulations with improved tolerability and bioavailability.
This review covers the immunopathophysiology of DED as well as the mechanism of action of cyclosporine A and its role in modulating ocular inflammation, compares the current ophthalmic cyclosporine A formulations, and discusses the limitations and challenges of using cyclosporine A for the treatment of DED.
Since Cyclosporine is a well-established stalwart of long-term management for dry eye disease (DED), Ophthalmologists have trusted it for years as an effective immunomodulator that alleviates dry eye symptoms without major negative side effects.
Cyclosporine Eye Drops
We have several options for cyclosporine: Cequa (0.09%, Sun Ophthalmics), Restasis (0.05%, Allergan) and the compounded Klarity-C Drops (0.1%, Imprimis).
Several more are in the pipeline, including generic Restasis, CyclASol (0.1%, Novaliq), Ikervis (0.1%, Santen Pharmaceutical) and OTX-CSI ophthalmic insert (0.36 mg, Ocular Therapeutix).
Although the concentrations range from 0.05% to 0.1%, we have found cyclosporine to be efficacious at all levels, with some subtle differences. The choice usually comes down to tolerability and affordability.
As a topical ophthalmic agent, cyclosporine poses several challenges. Firstly, it is a hydrophobic, lipophilic molecule, so does not easily penetrate the aqueous layer and reach the eye. Secondly, it can cause burning or stinging on instillation.
As a result, manufacturers need to formulate a vehicle that achieves the penetration required for efficacy, produces minimal side effects and feels comfortable for chronic use – a notoriously difficult feat.
The tolerability of different cyclosporine formulations hinges primarily on the vehicle, which varies among different products. Although we tend to favour products with an established track record of comfort, some patients might tolerate one better than another.
Because we have used Restasis—which has an anionic castor oil-in-water emulsion vehicle1 — for many years, we know to expect patients to be comfortable with its long-term use. As it has been around a long time, Restasis is also covered by most insurance plans.
Our patients have also tolerated Cequa well, which has a nanomicelle-based solution. Commercial patients can obtain Cequa at its lowest price through a specialty pharmacy, so we check with patients in advance to see if that is a convenient arrangement.
The third current option, Klarity-C Drops, has a chondroitin sulfate, glycerin and dextran-based solution. This can be the most cost-effective choice. However, in our limited experience, it also seems to be less well tolerated.
We use both cyclosporine and lifitegrast (Xiidra, Novartis) as part of long-term management plans. With cyclosporine, our best candidates have chronic dry eye and possibly wear contact lenses, so they need long-term relief from inflammation.
It can take up to 3 months for cyclosporine to build up the concentration required for full efficacy, so we tend to choose it for patients who can patiently await some improvement.
For more severe cases, we also utilise a steroid during the cyclosporine induction period, such as loteprednol etabonate ophthalmic suspension 0.25% (Eyesuvis, Kala Pharmaceuticals) which can be used on-label for short term treatment of dry eye.
In addition, our patients who have dry eye secondary to autoimmune conditions such as Sjogren’s syndrome or rheumatoid arthritis tend to respond very well to cyclosporine.
From a clinical standpoint, it has been great to see more options for long-term DED treatment. Whether we prescribe an immunomodulator or, optimally, offer some combination of therapies, the options allow ophthalmologists to tailor effective, economical and comfortable care.
Cyclosporine Eye Drops for Dogs
Cyclosporine eye drops for dogs are a valuable treatment option for various eye conditions in our furry companions. These drops are commonly prescribed to alleviate symptoms associated with conditions like dry eye syndrome, conjunctivitis, and keratoconjunctivitis sicca (KCS). Cyclosporine works by suppressing the immune response in the eyes, reducing inflammation, and promoting tear production. It helps to improve lubrication, relieve discomfort, and prevent further damage to the cornea. Cyclosporine eye drops for dogs are usually administered once or twice daily, as directed by a veterinarian. Regular use of these drops can significantly enhance the ocular health and overall well-being of our canine friends, ensuring their eyes stay comfortable and healthy.
Cyclosporine Eye Drops Price
The pricing of Cyclosporine eye drops can vary depending on various factors such as the brand, dosage strength, and quantity. Generally, Cyclosporine eye drops are available in both generic and branded forms, with generic options often being more affordable. It is advisable to consult with a healthcare professional or pharmacist to get accurate pricing information specific to your location and prescription. Additionally, insurance coverage and discounts may be available to help reduce the cost of Cyclosporine eye drops.
It is important to consider the potential benefits and effectiveness of the medication in relation to its price to make an informed decision about its affordability and value for your eye health needs.
(1).jpg)