Universal Treatment Approach May Be on Horizon for Retinitis Pigmentosa
In a landmark study that promises new hope for nearly 1.5 million people worldwide suffering from retinitis pigmentosa (RP), scientists at Columbia University Vagelos College of Physicians and Surgeons are pioneering a gene therapy that could potentially treat RP patients irrespective of their genetic mutation.
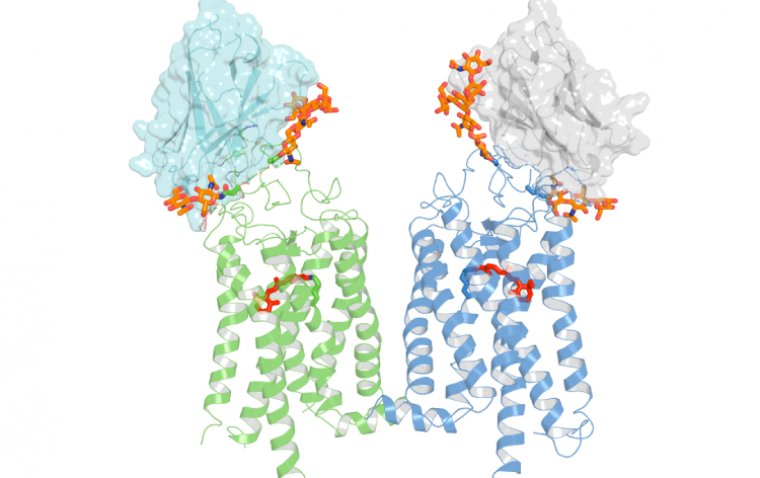
This groundbreaking approach leverages CRISPR-based genome engineering to correct a metabolic dysfunction across most RP variants, heralding a universal treatment strategy.
Overcoming Genetic Diversity in RP Treatment
Retinitis pigmentosa can be attributed to mutations in over 80 different genes, making the development of gene therapies for each variant a formidable challenge. However, the innovative treatment under development could overcome this obstacle by addressing a common metabolic error in the eye's photoreceptors.
Promising Early Results from Experimental Therapy
According to the research published in Cell Reports Medicine, the therapy, though still in the experimental phase and years away from human trials, demonstrated the ability to delay RP progression in mouse models by approximately one month, translating to an estimated ten years in human terms. This therapy proved effective across two genetically distinct RP models, suggesting wide applicability.
The significance of this approach cannot be overstated, as the current FDA-approved gene therapy for RP caters to a limited patient population in the United States, specifically those affected by mutations in the gene encoding the RPE65 isomerase.
"A universal precision metabolome rejuvenation would be a vast improvement over the limited options now available to most RP patients, which do little to prevent blindness," says study leader Stephen Tsang, MD, Ph.D., highlighting the therapy's potential to significantly enhance patient care.
Tackling the Clinical Challenge of RP
The clinical challenge of RP lies in the progressive energy starvation of the rods, the photoreceptors responsible for night vision. This degeneration subsequently affects the cones, crucial for daylight and color vision, leading to severe vision impairment or total blindness. The study proposes that enhancing glycolysis in rod cells can prevent or slow this degenerative process.
"By rejuvenating the glycolytic metabolome specifically within rods, we could slow down the aging of the retina and preserve vision," explains Nicholas Nolan, the study's first author. Unlike systemic treatments, the proposed CRISPR-based gene therapy targets only rod cells, minimizing potential side effects.
Next Steps Toward Clinical Application
The next steps involve further validation in rodent models before moving to larger animal studies, with the ultimate goal of translating these findings into a safe and effective treatment for humans. "We hope to extend the benefits of our gene therapy even further as we learn more about rejuvenating glycolytic metabolome and how it's affected by RP and aging," Tsang concludes, optimistic about the therapy's future and its implications for patients worldwide.
Reference
Nicholas D. Nolan et al, CRISPR editing of anti-anemia drug target rescues independent preclinical models of retinitis pigmentosa, Cell Reports Medicine (2024). DOI: 10.1016/j.xcrm.2024.101459
(1).jpg)