USC Roski Eye Institute Investigates Promising Therapies for TED
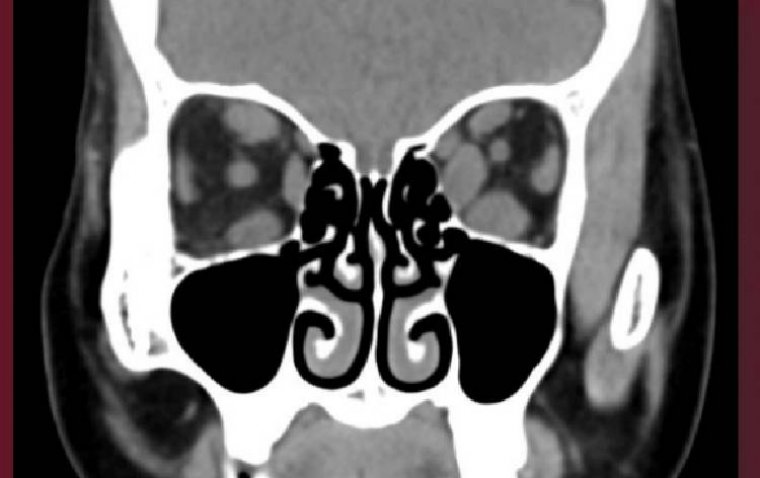
The USC Roski Eye Institute is making significant strides in the realm of Thyroid Eye Disease (TED) treatment through pioneering clinical trials exploring new medications. TED, a condition characterized by bulging eyes and potential vision impairment, has traditionally necessitated multiple invasive surgeries.
Dr. Sandy Zhang-Nunes, leading the trials at USC, elucidates the conventional approach, stating, "Traditionally, when treating TED, oculoplastic surgeons would do orbital decompression surgery... Then our strabismus colleagues would become involved to do eyelid surgery for double vision if needed. Eyelid surgery is often still needed as a third step."
Novel Drugs: A Beacon of Hope
The emergence of novel drugs offers hope for less invasive interventions. Dr. Zhang-Nunes explains, "New medications coming out mean the potential for less surgery, and even if surgery is still needed, it’s typically an easier surgery. It’s better for the patient."
Clinical Trials at USC Roski
At USC Roski, three distinct clinical trials for TED are underway, with more on the horizon. The first trial evaluated different dosages of an FDA-approved infusion medication over 52 weeks. Additionally, a second trial studying a different drug's efficacy recently concluded its high disease activity phase, while a third trial explores a drug with a unique mechanism of action.
Aim of the Trials
Ricardo Montoya, Clinical Research Coordinator at USC Roski, underscores the trials' aim: "We want to see if these TED investigational medications work on improving proptosis... Does it have less side effects? That’s the goal, for our patients to experience less side effects and improve the quality of their life as well as their vision."
Dr. Zhang-Nunes stresses the importance of personalized treatment plans due to potential variations in side effects among patients. The availability of multiple medication options enables tailored approaches to managing TED.
With promising results thus far, USC Roski Eye Institute remains committed to advancing the search for effective and minimally invasive TED treatments. The institute plans to continue recruiting participants for future trials, offering hope to TED patients worldwide.
(1).jpg)