Scientists Map Retinal Inhibitory Signaling System for the First Time
A team of international researchers has, for the first time, functionally mapped the smallest inhibitory signaling system of vision in mammals—unveiling a hidden control mechanism within the retina that could transform our understanding of visual processing and eye disease.
Decoding the Brain's Visual "Brake System"
Vision is one of the most intricate functions of the human brain, requiring a precisely coordinated interplay between numerous brain structures to process shapes, colors, depths, and motion. Underlying this process is a delicate balance of excitatory and inhibitory chemical signals in the retina—similar to how a car's accelerator and brake must work together for smooth driving.
The key inhibitory neurotransmitter in this process is GABA (gamma-aminobutyric acid). GABA serves as the brain’s primary “brake,” regulating nerve activity and maintaining equilibrium between excitation and inhibition within neural networks. Although extensively studied, the fine-scale architecture of GABAergic signaling in the retina—the eye’s first processing center for visual information—had remained unclear.
A Comprehensive Functional Map of Inhibitory Activity in the Retina
Now, researchers from DANDRITE (The Danish Research Institute of Translational Neuroscience) in collaboration with U.S. partners have conducted the first-ever functional mapping of inhibitory signaling within the retina. Their work focused on how GABA regulates the transmission of visual information from the eye to the brain.
Through this groundbreaking study, scientists mapped 44 distinct retinal cell types, including several that had not been previously described. Remarkably, the newly discovered cells revealed a higher degree of structural and functional organization than was previously recognized.
“Our result can be compared to finding a complicated map of an unknown landscape. Previously, we thought the landscape was randomly divided, but now we have mapped it, so we can see that each region has its own precise path and function,” explained Professor Keisuke Yonehara of DANDRITE.
Professor Keisuke Yonehara added,
“By understanding how the different cells in the retina are organized and work in specific directions, we have revealed how the brain can precisely 'see' movement and orientation in our visual field—as if they have found the most efficient route through a complex network.”
Technological Breakthrough: Real-Time GABA Sensor
The success of the study hinged on a newly developed GABA sensor, created by collaborators in the U.S. This sensor enabled researchers to visualize inhibitory neuron activity in real time, making it possible to capture and map the intricate dynamics of GABA signaling across retinal circuits with unprecedented precision.
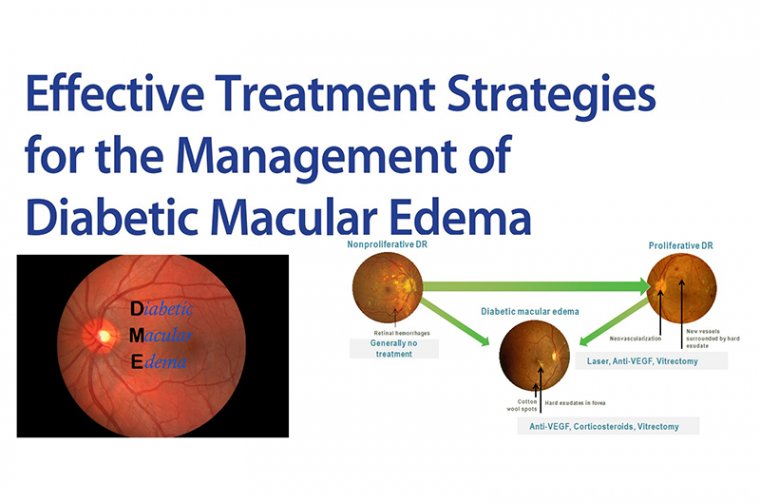
Implications for Eye Disease and Neurological Disorders
Published in Nature Neuroscience, the study offers significant implications for future ophthalmic and neurological research. According to Yonehara, this mapping effort could pave the way for deeper insights into the mechanisms behind eye disorders linked to inhibitory imbalance, such as congenital nystagmus, a condition characterized by involuntary, rhythmic eye movements.
“Many eye disorders are linked to imbalances in inhibitory signaling. Now we have a 'map' that can give us deeper insights into this condition and hopefully also others related to this area,” Yonehara noted.
Reference:
Akihiro Matsumoto et al, Functionally distinct GABAergic amacrine cell types regulate spatiotemporal encoding in the mouse retina, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-01935-0
(1).jpg)