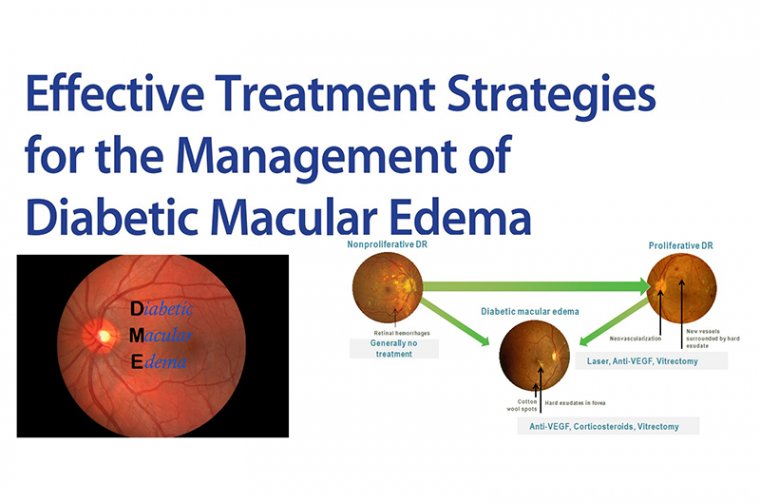
Diabetic Macular Edema & Treatment Strategies
Diabetic macular edema (DME) is the leading cause of blindness in the diabetic population.
Although its prevalence varies, the Diabetes Control and Complications Trial (DCCT) reported that 27% of type 1 diabetes (DM1) patients developed macular edema within nine years of onset.
Other studies indicate that in type 2 diabetic patients (DM2), prevalence increases from 3% within 5 years of diagnosis to 28% after 20 years.
DME tends to be a chronic disease, although spontaneous recovery is not uncommon. It is important to recognize that about 33% to 35% of patients resolve DME spontaneously after six months without treatment.
The disease is now believed to be multifactorial in origin with a number of systemic factors including hypertension, poor metabolic control of diabetes, dyslipemia and nephropathy playing a role in its pathogenesis.
Diabetic macular edema (DME) is a frequent cause of visual loss in patients with diabetic retinopathy (DR), and with the ever-growing number of patients with DME in clinical practice, there is an increased need for management protocols to provide better care for these patients.
There are different treatment strategies that can help in the management of diabetic macular edema and give patients better and stable visual outcome.
THE EFFECTIVE ROLE OF METABOLIC CONTROL ON THE RESPONSIVENESS TO TREATMENT OF DME
The differences between conventional and intensive diabetic therapies have been extensively studied, and a conclusion was made that an early intensive therapy does have a beneficial impact on long-term complications.
Proper intensive control significantly lowers the incidence of retinopathy progression and proliferative retinopathy compared with conventional therapy, despite no significant difference in mean glycated hemoglobin A1c (HbA1c) levels on the long-term follow-up (maintaining near-normal glycemic levels for an average of 6.5 years).
Initial 1% reduction in HbA1c at the time of initiating diabetic management lowers the risk of retinopathy by 30% to 40%.
Following DCCT2, when HbA1c levels in the intensive and conventional groups converged (year 8; INT, 7.98%; CON, 8.07%), the benefit of early intervention persisted with a 53% (P < 0.0001) reduction in the risk of further retinopathy progression, a 56% (P < 0.001) risk reduction in development of proliferative DR, and severe retinal outcomes and procedures to treat them were reduced by 50% in the intensive management group.
This is known as “metabolic memory,” which means in the long-term there is persistence of either the adverse effects of initial uncontrolled hyperglycemia or the beneficial effects of controlled hyperglycemia on the future development and progression of complications of diabetes.
Patients with extensive hard exudates on ophthalmoscopy with uncontrolled lipid profiles do usually benefit from anti-hyperlipidemic drugs. This will give a better response to injections themselves and decrease the recurrence of DME.
LASER THERAPY
Laser therapy has been considered the standard traditional therapy for diabetic eye disease for many years based on results from the ETDRS study.
Recently, there has been a decrease in the importance of retinal laser coagulation being a destructive procedure associated with irreversible morphological damage in the retina and resulting in many adverse effects.
Because a good metabolic profile is associated with improving the general diabetic condition, one may consider delaying the decision of irreversible laser therapy by choosing the intravitreal medical therapy as a trial to buy some extra time, and a chance to improve diabetic retinopathy and DME by simply becoming better controlled.
The 2 current indications for focal/grid macular laser for DME include: non-center-involving DME and ETDRS-defined DME (CS-DME).
Some retina specialists add laser to areas of focal thickening/areas of leakage; usually microaneurysms (MAs) as add-on therapy to injection, as this can decrease injection burden.
However, it has not been studied on a wide scale. Others use it as a rescue therapy once central macular thickness decreases to 400 microns to get an adequate response to injections.
Parameters using diode or argon laser include the smallest spot size (50-100 microns) you can obtain a minimal reaction with it (faint greyish) with minimal duration (0.05 -0.1 seconds) applied to the area outside the foveola (50-300 microns from the center).
Subthreshold (micropulse) diode laser has been tried in DME to minimize collateral damage by fragmenting the laser beam into micropulses, which can also be applied to the subfoveal area in a noticeably short duration combined with a longer interval (e.g., 5% duty cycle), allowing energy to be dissipated by the choroidal flow so it’s only stimulating the RPE.
No clinical trials are available to demonstrate superiority to conventional laser or anti-VEGF.
CORTICOSTEROID THERAPY
The FDA approved the use of steroids in DME due to their safety profile, efficacy, longer duration of effect, and cost-effectiveness. In situations in which anti-VEGF cannot be used (e.g., pregnancy or recent myocardial infarction), intravitreal corticosteroids are the drug of choice for resolving DME.
Intravitreal corticosteroids can be used intraoperatively in patients undergoing cataract surgery or postoperatively when the wound is stable enough.
It is better to start intravitreal steroids as a monotherapy and to closely monitor your patients for IOP elevations or other side effects for the first 6 weeks to 3 months.
Usually, if no significant rise in IOP occurs after the first injection, it is very unlikely to demonstrate a subsequent rise in IOP with further injections.
It may be important to consider the implant position role in triggering elevated IOP. In one study by Sudhakar et al., implants that remain close to the ciliary body/pars plana region (and the anterior segment) are associated with a higher probability of developing ocular hypertension and are more likely to require a triple drug therapy for IOP control.
VITRECTOMY
DME can have a tractional component caused by a thickened and taut posterior hyaloid clinically identified by the increased glistening of the paramacular vitreous face).
Initially advocated for clearing of media opacities and relief of retinal traction, vitrectomy techniques have advanced, leading to more complex indications for the treatment of DME.
Vitrectomy facilitates greater blood flow through retinal vessels, and it has also been reported to improve retinal oxygenation 10 times more than in non-vitrectomized eyes.
A review of previous findings shows that vitrectomy is effective at reducing retinal thickness in some cases of chronic DME. However, vision fails to improve in many of these patients.
The reason may be that only recalcitrant DME cases—such as those with permanent outer retinal damage—that have failed other therapies are undergoing vitrectomy.
In many of these studies, investigators did not employ optical coherence tomography (OCT) when evaluating their DME patients.
The integrity of the external limiting membrane and the inner segment/outer segment, disorganization of the retinal inner layers, size of the foveal avascular zone, and the presence of hyperreflective foci are all significant prognostic indicators.
Parallelism—the measure of how straight and parallel the retinal layers are—is a quantifiable measure of retinal layer integrity and can be used to forecast which patients can benefit from surgery.
A pars plana vitrectomy (PPV) can be performed with or without peeling of the internal limiting membrane (ILM).
However, there is prospective evidence to suggest that ILM removal during PPV with posterior vitreous detachment (PVD) induction is more effective for stabilizing vision and improving cystoid macular edema than PPV and PVD alone.
We suggested that after adjusting for the baseline VA, the older age, associated with a more severe DR on clinical examination, the presence of surface wrinkling retinopathy on fundus photographs, and the previous pan-retinal photocoagulation (PRP) treatments were associated with a poor benefit on VA over 1 and 2 years.
Another indication for vitrectomy is media opacity precluding proper evaluation and management, including proper photocoagulation. Vitrectomy is an alternative approach to the treatment of recalcitrant DME.
DME AND WET AMD
Although it is uncommon in clinical practice, double pathology can exist, and a patient who has been treated for DME can live long enough to develop CNV due to wet AMD or other neovasculopathies.
Although it has been reported that diabetic retinopathy can be a protective factor against the development of AMD, there has been much debate on this issue.
Management is likely to stay the same (injections), but you must discuss the prognosis with your patient.
PATIENT COMPLIANCE
One of the major challenges that we face in the management of diabetic eye disease is patient adherence to treatment and follow-up schedules. Diabetic retinopathy-related vision loss is preventable with proper screening and timely treatment.
We recommend that patients with diabetes receive a dilated fundus exam every year, but the overall compliance rate for this annual screening recommendation is only 50-60%, according to published data.
This is a major issue, especially tragic when compliance becomes a factor in someone’s disease progression. Loss to follow-up (LTFU) rates after anti-VEGF injections for DME (defined as not returning for a visit within 12 months after an injection) have been found to be shockingly high—around 25%.
Unfortunately, LTFU happens all the time in clinics, and it has worsened during the COVID-19 pandemic period.
Diabetic macular edema represents a significant public health challenge. Many patients are undiagnosed and untreated, and even those treated with standard therapy may respond poorly and progressively lose vision.
Insight into the pathophysiology of DME has led to novel treatments, including anti-VEGF and corticosteroid-based treatment strategies.
Drug delivery into the vitreous cavity remains an important limitation of many of these new treatments, as the risks of endophthalmitis, retinal detachment, and other adverse events become cumulative with repeated intravitreal injections.
Injectable and/or implantable drug delivery devices may offer the benefits of chronic therapy, while reducing the adverse events associated with repeated drug delivery. Patient-centered therapeutic algorithms should be considered to achieve satisfactory management of DME.
Factors predicting a better response to the treatment have been reported, including younger age, a good controlled systemic condition, and a better baseline functional and anatomical ocular condition, such as visual acuity, retinal thickness, and photoreceptor’s integrity.
Although a consensus-based treatment for DME associated with macular ischemia is still lacking, the strategies outlined in this article are critical to better patient outcomes.
(1).jpg)