New Dual-Modality Imaging System Offers High-Resolution Retinal Oxygen Mapping
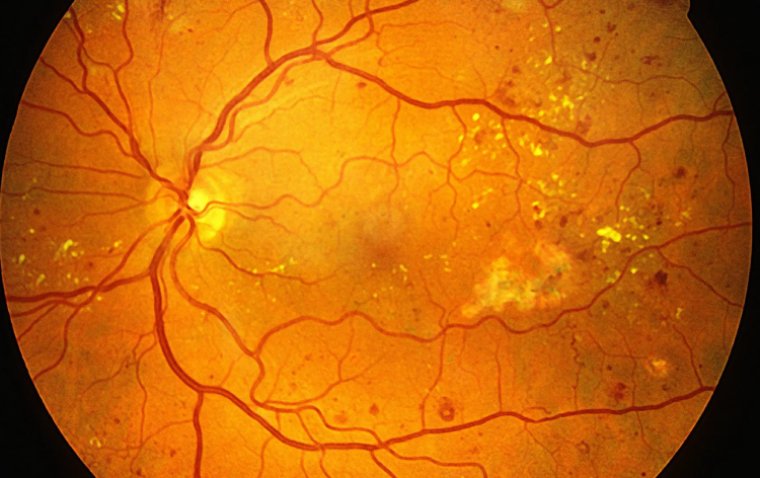
Researchers from Johns Hopkins University and the University of Pennsylvania have developed a pioneering imaging system that captures both retinal microstructure and oxygen metabolism with unprecedented detail. Reported in Neurophotonics, the system integrates visible light optical coherence tomography (VIS-OCT) with phosphorescence lifetime imaging scanning laser ophthalmoscopy (PLIM-SLO), enabling real-time, spatially aligned insights into the retina’s oxygen dynamics.
Advanced Imaging: Structure and Oxygen Metabolism Combined
The novel system combines two complementary optical techniques:
• VIS-OCT: Delivers high-resolution, 3D visualization of retinal microanatomy and blood flow.
• PLIM-SLO: Measures oxygen partial pressure (pO₂) in retinal blood vessels by detecting phosphorescence lifetime changes in a molecular probe.
To obtain oxygenation data, researchers injected Oxyphor 2P, a specialized phosphorescent probe, into mice. This molecule alters its light emission characteristics depending on local oxygen levels. When paired with controlled light pulses, the system calculates highly specific pO₂ values down to individual capillaries.
Precise Retinal Oxygenation Mapping in Live Animal Models
By synchronizing VIS-OCT and PLIM-SLO within a shared optical path, the team ensured that oxygen readings and structural images were spatially co-registered. This allowed detailed assessment of oxygen distribution across different vessel types and retinal depths.
Key findings from healthy mice included:
• Arterioles showed higher pO₂ than venules, as expected.
• Capillary pO₂ levels aligned with systemic oxygen saturation.
• The system produced oxygen dissociation curves consistent with known hemoglobin physiology.
• Oxygenation changes were successfully tracked in response to altered inhaled oxygen levels.
Implications for Retinal Disease Research and Diagnostics
The dual-channel imaging platform presents a non-invasive, repeatable method for longitudinal studies in mouse models of retinal disease, such as diabetic retinopathy or age-related macular degeneration. By mapping oxygenation and structure simultaneously, researchers can better understand how microvascular dysfunction contributes to vision loss and disease progression.
Importantly, this technique supports the development and validation of label-free oximetry using VIS-OCT, by comparing it to the gold-standard PLIM-SLO measurements.
Future Directions
The research team notes that the system may be further enhanced by incorporating adaptive optics to sharpen image resolution. Such refinements could support clinical translation, offering a foundation for next-generation tools in the diagnosis and monitoring of human retinal diseases.
Reference:
Stephanie Nolen et al, Multimodal retinal imaging by visible light optical coherence tomography and phosphorescence lifetime ophthalmoscopy in the mouse eye, Neurophotonics (2025). DOI: 10.1117/1.nph.12.3.035015
(1).jpg)