Latest Medications For Dry Eye Disease Treatment
Dry eye disease includes a group of conditions in which the eye does not produce an adequate volume of tears or when the tears are not of the correct consistency.
The chance of experiencing dry eye increases with age, affecting approximately five percent of the adult population age 30-40 and 10 to 15 percent of adults over age 65, and is more common among women.
When severe and left untreated, this condition can lead to pain, ulcers or scars on the part of the eye called the cornea.
Dry eye can make it more difficult to perform some activities, such as using a computer or reading for an extended period of time, and it can decrease tolerance for dry environments, such as the air inside an airplane.
Over the last 18 months, rapid developments have occurred in the dry eye and ocular surface disease space.
There are many approaches and algorithms to treat inflammation and meibomian gland obstruction, most of which are too complicated for even the most experienced premium surgeons.
The good news is that newer products are arriving to help keep the riffraff away from the ocular surface sooner in the process. In our opinion, these newer approaches may be the start of curing dry eye disease altogether.
Dry eye (DED) is a ubiquitous ophthalmic disease that will inevitably increase in incidence worldwide as the population continues to age.
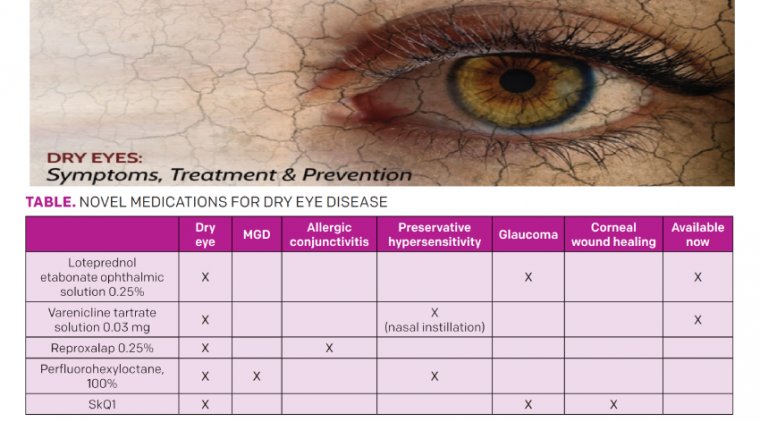
A complex, multifactorial disease, dry eye commonly coexists with meibomian gland disease, allergic conjunctivitis, glaucoma and preservative hyper-sensitivity. Dry eye can also be induced or exacerbated by surgical interventions.
As novel medications with vastly different mechanisms of action are developed to address nuanced forms of dry eye, practitioners will have more tools to address clinical signs and symptoms of dry eye in their patients.
Topical steroid therapy exemplified the very earliest efforts to abate inflammation, an important dry eye mechanism. This led to the approval of Eysuvis (loteprednol etabonate ophthalmic suspension, 0.25%, Kala Pharmaceuticals) in late 2020.
With a novel mucus-penetrating particle vehicle, it was the first steroid specifically approved for episodic dry eye. A patient prone to glaucoma may best be served by either loteprednol, a selective ester steroid with significantly less proclivity to IOP elevations, or another class of agent altogether.
Another new-to-market medication, Regener-Eyes, is a preservative-free biological eyedrop that uses d-MAPPS (derived-Multiple Allogeneic Proteins Paracrine Signaling) technology to stimulate stem cell communication without cell contact.
This option is usually prescribed for mild-to-moderate or severe DED, especially when there is corneal involvement such as punctate epithelial keratitis.
Recently, a variety of other novel medications, highlighted in part herein, have been developed to address additional factors implicated in DED.
TYRVAYA - Varenicline tartrate solution 0.03 mg (Tyrvaya, Oyster Point Pharma), approved in late 2021, is a novel dry eye treatment that avoids eyedrops altogether.
Investigated through the MYSTIC Phase 2 randomized trial and the ONSET-2 Phase 3 randomized trial, Tyrvaya has repeatedly been shown to significantly improve signs and symptoms of dry eye.
The primary endpoint in the MYSTIC trial was mean change from baseline in Schirmer’s score at day 84 and for ONSET-2 improvement in Schirmer’s test by 10 mm or more at week 4; however, tear production was shown to increase as quickly as 5 minutes after administration.
While the exact mechanism of action is not completely understood, Tyrvaya is thought to be a neuro-stimulating agent, activating the parasympathetic pathway of the nasociliary branch of the trigeminal nerve in the nose, thereby increasing baseline tear production.
The active ingredient in Tyrvaya, varenicline, has also been used as a smoking cessation aid. Given as an oral tablet in much greater systemic doses as Chantix, varenicline has an excellent tolerance.
The side effect profile of Tyrvaya is relatively benign, with the most common adverse reaction being sneezing, which occurs in 82% of patients.
Additional adverse reactions include cough, throat irritation and instillation-site (ie, nose) irritation. Given by nasal spray twice daily in each nostril, Tyrvaya can not only reduce treatment burden for patients, but is also an excellent option for patients who have difficulty with drops.
For example, patients with ocular toxicity, reduced neck mobility or upper limb mobility, tremors, digital arthritis, who live alone or who struggle with self-administering eyedrops may all benefit from dry eye treatment via nasal spray.
In addition, Tyrvaya provides a solution to the challenging glaucoma patient on multiple medications to lower IOP or preserve filtration surgery, as they may not welcome another drop for what might be their secondary diagnosis of dry eye.
Nasal delivery mitigates the compliance, toxicity and nuisance factors of burgeoning medicamentosa. Of note, Tyrvaya was not tested on patients who use CPAP, with prior sinus surgeries, with a history of PKP and those with recurrent nosebleeds.
As such, no conclusions can be drawn about its effectiveness in these specific demographics. Nevertheless, the approval of both a new delivery route and a novel mechanism of action in one new pharmaceutical portends subsequent exciting developments for dry eye patients.
REPROXALAP - Reproxalap (Aldeyra Therapeutics) is a new topical therapeutic currently being developed to address both allergic conjunctivitis and DED.
Reproxalap is a novel, small-molecule immune-modulating covalent inhibitor of reactive aldehyde species (RASP). While the exact mechanism of action is not fully understood, RASP potentiates inflammation through a variety of inflammatory mediators and pathways.
RASP inhibitors are the gatekeepers of inflammation. They work quickly and efficiently at the top of the inflammatory cascade before other drugs like steroids and immunomodulators that work downstream in the inflammatory process, before T cells become activated and before cytokines are released.
As such, Reproxalap presumably reduces inflammation implicated in both allergic disease and dry eye as was demonstrated in Phase 2b and Phase 3 trials.
Phase 2b clinical trials demonstrated rapid, broad and clinically relevant symptomatic control, in conjunction with statistically significant improvement over vehicle in signs of DED as demonstrated by fluorescein staining, in DED patients over 12 weeks of therapy.
In a Phase 2b trial, patients were randomized to reproxalap 0.1%, reproxalap 0.25% and placebo. Participants were then exposed to a controlled adverse environment, consisting of low humidity, for 90 minutes after a 12- week course of q.i.d. treatment.
Relief for symptoms of dry eye was appreciated as early as 2 weeks into the treatment course, at the first follow-up visit.
Patients experienced symptomatic improvement in a dose-dependent response, notably relative to grittiness and dryness. Researchers also appreciated improvements in nasal fluorescein staining in the 0.25% group.
For patients with allergic conjunctivitis, Reproxalap was evaluated in a randomized, double-blind Phase 2 trial and in the Phase 3 ALLEVIATE trial.
In each of these studies, participants were randomized to various treatment groups, including reproxalap 0.25%, reproxalap 0.5% and vehicle. Participants who had been administered reproxalap noted improvement in symptoms including tearing, itching and redness.
Higher doses of reproxalap (0.5% vs 0.25%) were associated with higher rates of redness after their first dose, suggestive of some instillation site reaction (ie, eye irritation).
Aldyera selected a 0.25% regime to develop, given the clinical relevance observed with the improvement in ocular itching and conjunctival redness. A new drug application submission for reproxalap is anticipated later this year.
This is a promising medication for those suffering from both dry eye and allergic conjunctivitis. Typically, muscarinic topical antihistamines dry the ocular surface, while topical therapies for dry eye and meibomianitis frequently create hypersensitivity or irritative effects on the allergic ocular surface.
No class of agents other than steroids currently simultaneously serve these demographics, so a truly game-changing therapeutic for millions of patients with both conditions will be welcomed.
NOV03 - In 2019, Bausch Health announced that it licensed Novaliq’s NOV03 investigational treatment for commercialization and development in the United States and Canada. NOV03 is a unique medication that has been demonstrated to address both DED and MGD.
The active ingredient in NOV03, perfluorohexyloctane (F6H8), is a preservative-free ophthalmic solution that stabilizes the lipid component of the tear film and penetrates meibomian glands.
Within the meibomian glands, the perfluorohexyloctane interacts with and assists with dissolving the viscous meibum in the glands.
In the double-blind randomized Phase 2 saline-controlled trial SEECASE, NOV03 demonstrated improvement in both signs and symptoms of dry eye, specifically in total fluorescein staining, tear break-up time and in participant responses to the eye dryness score.
These improvements can begin to be appreciated as early as 2 weeks into the full 8-week treatment.
A low rate of instillation site reaction (ie, eye irritation) was reported in patients in a dose-dependent response, in 2.6% of patients receiving q.i.d. and 0.9% of those receiving b.i.d. Australia, New Zealand and Europe have approved equivalent ophthalmic drops, 100% perfluorohexyloctane, with an excellent safety profile.
An additional consideration is that trials did not include patients on topical glaucoma medications, and, as such, interactions with additional topical ocular hypotensives have yet to be investigated.
The semi-fluorinated alkane molecule class is also an outstanding water-free vehicle capable of carrying a wide variety of active pharmaceutical ingredients, including cyclosporine A 0.1% found in the Novaliq CyclASol preparation, now in a confirmatory ESSENCE II Phase 3 randomized controlled trial.
In addition to water-free, preservative-free properties, the 20 l drop size reduces drop overflow. The CyclASol clinical data shows tolerability similar to vehicle.
VISOMITIN -- Mitotech has been investigating a novel medication, Visomitin (SkQ1), a cardiolipin peroxidase inhibitor. Previously investigated in relation to senesce and neurodegenerative conditions, SkQ1 has demonstrated promising results relative to the ocular surface as well.
SkQ1 has a novel mechanism of action as a mitochondrial-targeted antioxidant, addressing the oxidative stresses that occur in both DED and corneal wounds. As such, SkQ1 holds promise for dry eye that occurs postoperatively after cataract extraction.
Investigation into SkQ1 is ongoing. In the randomized, double-blind, placebo-controlled Phase 3 VISTA-2 trial, SkQ1 is being studied to treat moderate to severe DED. Primary endpoints in this study include improvement in central corneal staining, BCVA and eye discomfort.
The tolerance of SkQ1 has been excellent, with a side-effect profile similar to that of an artificial tear. SkQ1 formulations do contain preservatives. While the Phase 3 trial is ongoing, SkQ1 holds excellent potential for patients with dry eye exacerbated by such events as cataract surgery.
Interestingly, mitochondrial antioxidants can also lower IOP. Commonly, glaucoma patients administering multiple vision-saving topical ocular hypotensive agents develop recalcitrant ocular surface disease.
A dual mechanism controlling both dry eye and glaucoma would benefit a large, currently underserved patient base.
Dry eye is a complex, multifactorial disease that often coexists with other ocular and systemic pathology. Ongoing research has demonstrated great promise in the treatment of dry eye in new, innovative ways, while simultaneously addressing concomitant pathology.
With this burgeoning research in the nuanced presentations of dry eye and related pathology, practitioners are now better able to assist patients in finding a solution that can provide relief.
(1).jpg)