Intraocular (Uveal) Melanoma Treatment
The mean age-adjusted incidence of uveal melanoma in the United States is approximately 4.3 new cases per million people, with no clear variation by latitude. Men have a higher incidence at 4.9 cases per million than do women at 3.7 cases per million.
The age-adjusted incidence of this cancer has remained stable since at least the early 1970s. U.S. incidence rates are low compared with the rates of other reporting countries, which vary from about 5.3 to 10.9 cases per million.
Some of the variation may be the result of differences in inclusion criteria and methods of calculation.
Uveal melanoma is diagnosed mostly at older ages, with a progressively rising, age-specific incidence rate that peaks near age 70 years.
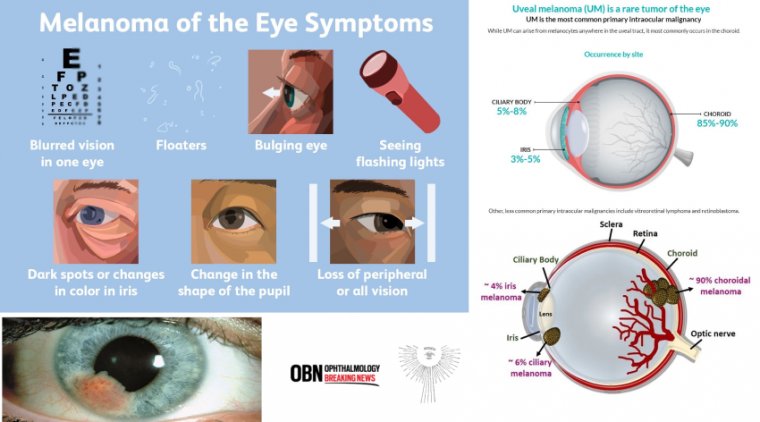
Ocular melanoma is the most common primary cancer of the eye in adults. It occurs most often in lightly pigmented individuals with a median age of 55 years. However, it can occur in all races and at any age.
Called "OM" for short, ocular melanoma is a malignant tumor that can grow and spread to other parts of the body - this process, known as metastasis, is often fatal and occurs in about half of all cases.
Although produced from the same cells in the body, called melanocytes, OM is different from skin (or cutaneous) melanoma and is not related to sun exposure. Ocular Melanoma is the second most common type of melanoma after cutaneous and represents about 5% of all melanomas.
Similar to melanoma of the skin, OM is a little understood and silent killer. In the majority of cases, ocular melanoma develops slowly from the pigmented cells of the choroid (choroidal melanoma) but it also can develop from the pigmented cells of the iris and ciliary body.
It is also called uveal melanoma because the uvea is a part of the eye containing two areas in which OM can commonly occur.
OM is an aggressive form of cancer that can involve any of three areas of the eye: the iris (the pigmented area surrounding your pupil), the ciliary body (a thin tissue layer in your eye responsible for aqueous humor production), and/or the choroid or posterior uvea (the vascular layer of the eye between the retina and the white outer layer known as the sclera; this pigmented tissue full of blood vessels nourishes the retina).
These three areas are collectively known as the uvea or uveal tract, and OM can occur in any combination of the three. Iris melanomas have the best prognosis, whereas melanomas of the ciliary body have the worst.
Most uveal tract melanomas originate in the choroid; the ciliary body is less commonly a site of origin, and the iris is the least common. Melanoma can also occur in the thin lining over the white part of the eye (the conjunctiva) or on the eyelid, but this is very rare.
OM tumors arise from the pigment cells (melanocytes) that give color to the eye. Formation of these tumors is quite rare and, as for many other forms of cancer, the exact cause is unknown.
It is known that exposure to ultraviolet (UV) rays (either from the sun or sunbeds) increases the risk of developing melanoma of the skin.
People whose skin burns easily are most at risk – people with fair skin, fair or red hair and blue eyes. However, there has no conclusive evidence linking UV exposure and OM.
LIGHT-ACTIVATED AU-011 has the potential to be the first targeted therapy ever developed for the primary treatment of ocular melanoma, the most common primary cancer of the eye, said Amy C. Schefler, MD, with Retina Consultants of Houston.
People diagnosed with ocular melanoma confront “an array of poor treatment options, which often result in severe vision loss, removal of the eye, and in about half of all cases, metastasis to the liver, where the disease is nearly always fatal,” according to Aura Biosciences, which developed this light-activated technology.
AU-011 is a first-in-class targeted therapy, a novel protein capsid-dye conjugate recombinantly derived from the capsid proteins of the papilloma virus; the FDA has already granted Fast Track Designation and Orphan Drug Designation, recognizing that there are no FDA-approved therapies and that the disease is serious and life-threatening, Dr. Schefler said.
The mechanism of action of AU-011 is targeted acute tumor cellular necrosis upon light activation. The capsid conjugates are delivered by intravitreal injection and selectively target the tumor cells in the choroid sparing the retina and other key ocular structures.
This unique selectivity is due to the binding of the capsid conjugates to modified heparan sulfate proteoglycans (HSPGs) that are expressed on the tumor cell surface.
The capsid protein is conjugated with a potent phthalocyanine photosensitizer, IRDye 700DX, that exerts its tumor cytotoxic effect through light-activation with a near-infrared 689-nm laser.
The mechanism of action has a dual selectivity. First, the capsid conjugates bind tumor cells selectively without binding other key eye structures and second, the laser beam activates the conjugated dye exclusively within the tumor generating potent cytotoxic oxygen free radical species that disrupt the tumor cell membrane, leading to targeted and acute tumor cellular necrosis, Dr. Schefler said.
In preclinical ocular tumor rabbit models, animals were treated with two weekly doses of light activated AU-011 on day 1 and day 8. The results demonstrated complete necrosis in the tumors implanted in the choroid with no damage to the adjacent retina even in tumors measuring >10mm in thickness, Dr. Schefler said.
The animal data showed a dose response effect, which means that with higher doses of the drug the tumor necrosis increased, while the retina and other healthy tissue were not affected, she said.
The strength of this preclinical data enabled the Orphan Drug Designation by the FDA in 2015. Dr. Schefler presented the interim 6-month results on AU-011.
Study Design
The phase Ib/II trial is a 2-year, prospective, multicenter, open label design with single and multiple ascending dose cohorts, she said.
Three patients with choroidal melanoma received a single intravitreal administration of the capsid conjugate at each of three subtherapeutic dose-escalating levels (20 μg, 40 μg, or 80 μg) followed by light-activation with a 689-nm laser at a fluence of 50 J/cm2 .
The multiple ascending dose (MAD) phase of the study consisted of four additional cohorts of three patients each.
In each cohort the number of intravitreal administrations was increased up to three weekly treatments and the number of laser activations was increased to two applications on the same day, separated by 30 minutes.
The objective of the MAD was to explore the maximum tolerated dose and regimen for the phase III clinical trials. The primary trial objective was safety, with secondary outcomes including efficacy and immunogenicity.
Study Results to Date
There have been 22 patients treated to date, nine in the single ascending dose cohort, and 13 in the multiple ascending dose cohort. “There were no treatment-related serious events or severe adverse events,” Dr. Schefler said.
There were no dose-limiting toxicities observed. Adverse events were manageable with standard of care treatments and had no further clinical sequelae. Pre-treatment visual acuity was maintained in all subjects that have been followed for 6 to 12 months, Dr. Schefler said.
All adverse events were deemed mild to moderate, and included anterior or posterior inflammation (in 10 and 11 patients [n = 18], respectively), which started about 4 weeks after treatment. There was transient increased intraocular pressure in 6/18 patients.
Of note, the inflammation and the IOP increase were manageable to date with standard treatment and resolved without clinical sequelae.
“Interestingly, inflammation is an expected response to the mechanism of action of AU011 as a result of the acute tumor cell necrosis, and may actually be beneficial in terms of creating a tumor-specific T-cell immune response,” she said.
“Rather than prevent this up front, we want to treat it once it happens.” Fundoscopic evaluation of patients after treatment showed that the posterior inflammation appears to start in the tumor and “is probably related to tumor necrosis.”
There was virtually no change in visual acuity (all patients followed for 6 months or longer were within one letter of baseline). “This was regardless of where the tumor was located, including those that were right in the middle of the fovea or touching the optic nerve,” she said.
In the three single-dose cohorts, several patients had stable disease at 12 months, even though this had been considered the sub-therapeutic dose, Dr. Schefler said.
In the multiple dose cohorts, all patients maintained stable disease at follow-up and a number of patients had tumor shrinkage measured by ultrasound.
Dr. Schefler said investigators are measuring tumor growth, and presuming that when there is no further growth the tumor is undergoing necrosis with no malignant potential any longer.
“Certainly, seeing tumor shrinkage and inflammation around the tumor at early time points is encouraging to confirm the biological activity of the drug, but the most important measure for efficacy is that the tumors have no further growth at longer follow up times with good vision preservation,” Dr. Schefler said.
(1).jpg)