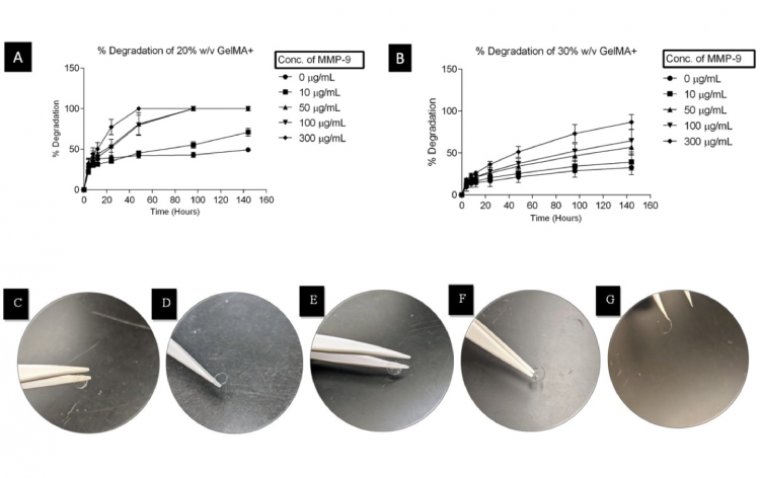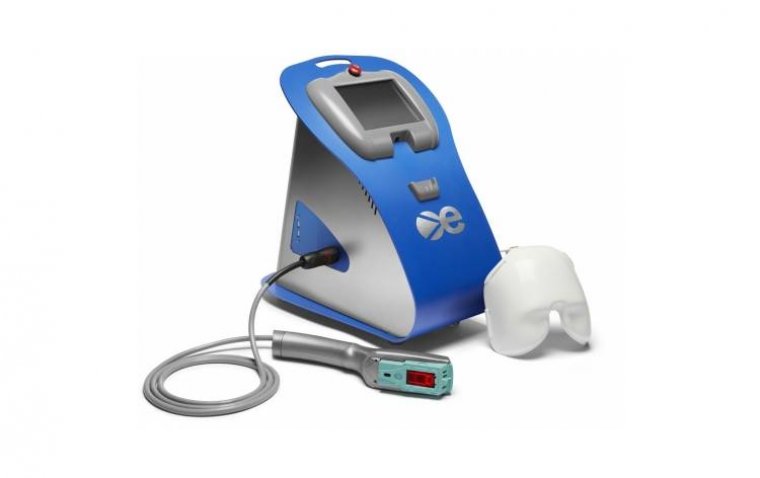
Eye-light® Device Receives CE Mark for Dry AMD and Extended Anterior Segment Pathologies
Espansione Group announced that it has received CE (MDR) approval for its flagship device, eye-light®, covering a broad spectrum of conditions, including both new retinal and corneal pathologies.
The eye-light® device boasts various photobiomodulation terminals optimized for treating a diverse range of conditions, now expanded to include anterior and posterior segment pathologies. This clearance includes treatments for chalazion, blepharitis, demodex-related conditions, rosacea, post-blepharoplasty complications, and age-related macular degeneration (AMD).
Positive Results from LightWave I Clinical Trial
The announcement came after positive results from the LightWave I clinical trial, focusing on dry AMD, presented at the ARVO 2024 Congress in Seattle, USA.
Alessandro Fier, Executive Board Member & CEO, commented: “Espansione has been the first player to develop and obtain clearance of a photobiomodulation device in ophthalmology, almost a decade ago, revolutionizing dry eye treatment. These new clearances enable us to offer an unmatched portfolio of effective treatments on our tech platforms to European eye care professionals. This milestone validates Espansione as a key innovator in the ophthalmic medical technologies arena, and is a significant step towards our long-term vision of becoming the global leader in photobiomodulation."
Matteo Corbellino, Executive Board Member and Chief Marketing & Innovation Officer, stated: “This outstanding milestone is a testament to the team’s relentless focus on clinical development and the strong bond we share with the broader research community, for which we’re deeply thankful—without it, this achievement would never have been possible.” adding also that “This achievement comes out of a couple of decades of work in this field and is a testament to the potential our technology holds. We’re certain it will pave the way for future discoveries and innovations that will make photobiomodulation an essential tool for Eye Care Professionals and other medical specialists.”
Global Expansion: Espansione Group's Ambitious Plans
Beyond the EU27 countries, Espansione Group plans to extend these novel treatments to other regions, including EMEA, LATAM, and APAC countries.
With a rich history in light-based healthcare technologies, Espansione Group enhances the value of its portfolio, including Light Modulation® Low-Level Light Therapy (LM® LLLT) and Optimal Power Energy® Intense Pulsed Light (OPE® IPL), available through solutions like eye-light®, meibomask® / mgd-mask®, and my-mask®.
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
Reference:
(1).jpg)