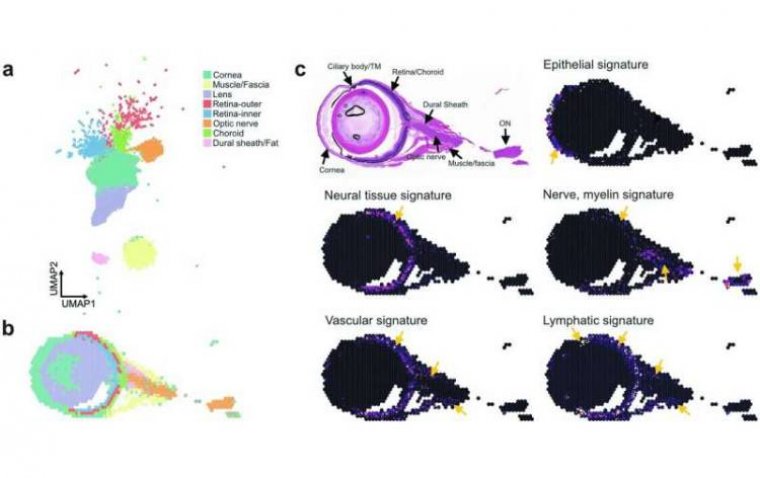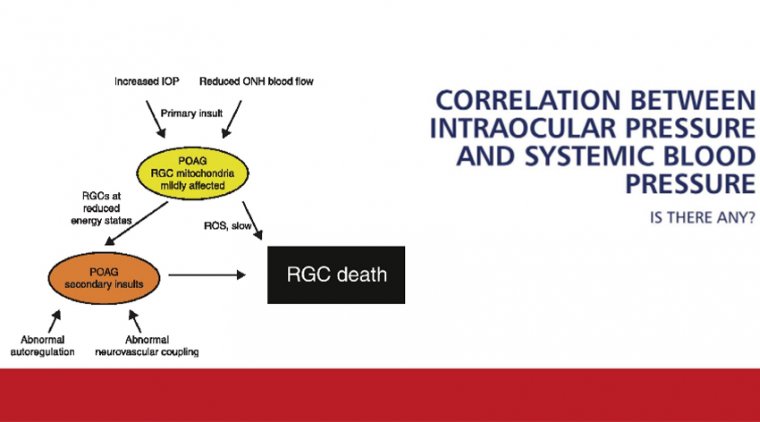
Elevated Intraocular Pressure Linked to Vision Loss and Optic Nerve Damage, Study Finds
Elevated eye pressure (ocular hypertension) may play a more direct role in irreversible vision loss than previously understood, according to a study published in Ophthalmology Science. The research highlights the mechanical and vascular consequences of elevated intraocular pressure (IOP), potentially offering new directions for early glaucoma detection and treatment.
Investigating the Impact of Ocular Hypertension on Blood Flow
Led by Yi Hua, PhD, a biomedical engineering professor at the University of Mississippi, in collaboration with researchers at the University of Pittsburgh, the study explored how elevated IOP affects the eye’s vascular system.
“We wanted to see how intraocular pressure changes and deforms the blood vessels in the eye,” said Hua. “If we can understand that, we can inform drug delivery to improve blood flow in the back of the eye. That can slow down the progression of glaucoma.”
Glaucoma and Hypoxia: A Vascular Perspective
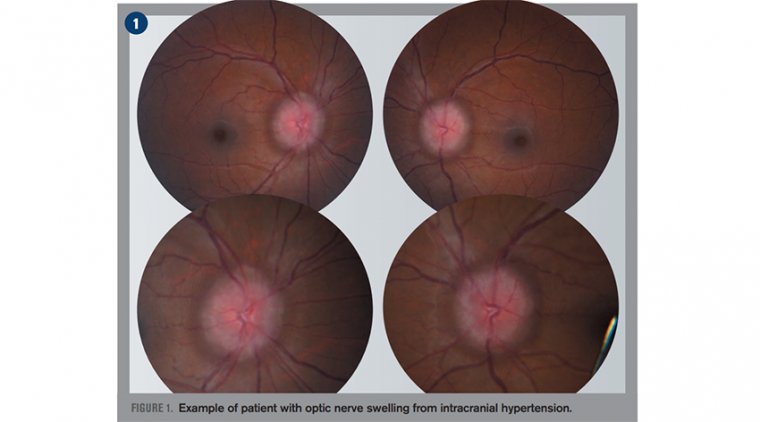
Glaucoma, a leading cause of irreversible blindness worldwide, is often asymptomatic until significant optic nerve damage occurs. The study demonstrated that elevated IOP can compress the lamina cribrosa—a mesh-like structure in the optic nerve head—thereby constricting blood vessels and reducing oxygen supply to critical ocular tissues.
“This can lead us to a new way to diagnose glaucoma earlier,” said Yuankai Lu, PhD, postdoctoral researcher at the University of Pittsburgh and co-author of the study. “If this finding holds true, then we can use blood flow supply to predict the development of this disease.”
Using 3D modeling and fluorescent dye imaging, the researchers visualized how blood flow responds to varying levels of pressure. Notably, even mild increases in IOP caused vascular deformation and localized hypoxia. In cases of extreme pressure, up to 30% of the lamina cribrosa tissue experienced oxygen deficiency.
Beyond Pressure Reduction: A Need for Novel Therapies
“We still do not have an efficient way to slow down the progression of glaucoma,” said Hua. “The only current option is to reduce eye pressure. But for some patients, even after pressure is lowered, damage continues. So, we need better methods.”
Ian Sigal, PhD, associate professor of ophthalmology and bioengineering at the University of Pittsburgh, emphasized the long-term impact of chronic IOP elevation:
“The eye can weather a short-lived increase in eye pressure. But a chronic increase over weeks, months, or years can cause substantial damage. The vision loss resulting from this damage cannot be recovered.”
Advancing Glaucoma Research with Imaging and Modeling
Unlike previous studies that relied mainly on statistical associations, this study combined advanced imaging techniques with computational modeling, offering a more mechanistic understanding of how elevated IOP leads to vascular insufficiency and neural damage.
“Most glaucoma research is based on statistics, which can give you a correlation,” said Lu. “By combining imaging techniques with 3D modeling, we gained a more comprehensive understanding of blood flow and oxygen distribution in the eye.”
Early Detection Remains Critical
The researchers stress that early detection and regular eye exams are essential, especially for those at increased risk of glaucoma, including individuals with:
• High blood pressure or diabetes
• A family history of glaucoma
• Higher prevalence among Black and Latino populations
“We really want to raise awareness of this issue,” Hua said. “A lot of people know the risk of high blood pressure, but we want to also raise the importance of elevated eye pressure.”
Reference:
Yuankai Lu et al, Impact of Elevated Intraocular Pressure on Lamina Cribrosa Oxygenation: A Combined Experimental–Computational Study on Monkeys, Ophthalmology Science (2025). DOI: 10.1016/j.xops.2025.100725. www.ophthalmologyscience.org/a … (25)00023-5/fulltext
(1).jpg)