CorNeat Vision Launches CorNeat EverPatch in the U.S.
CorNeat Vision has launched the CorNeat EverPatch in the United States, a novel synthetic tissue-integrating matrix poised to transform ophthalmic surgeries. Endorsed by leading surgeons across the US, this FDA-cleared device marks a significant advancement in addressing complications associated with surgical interventions on the ocular surface.
Unmatched Advantages Over Traditional Grafts
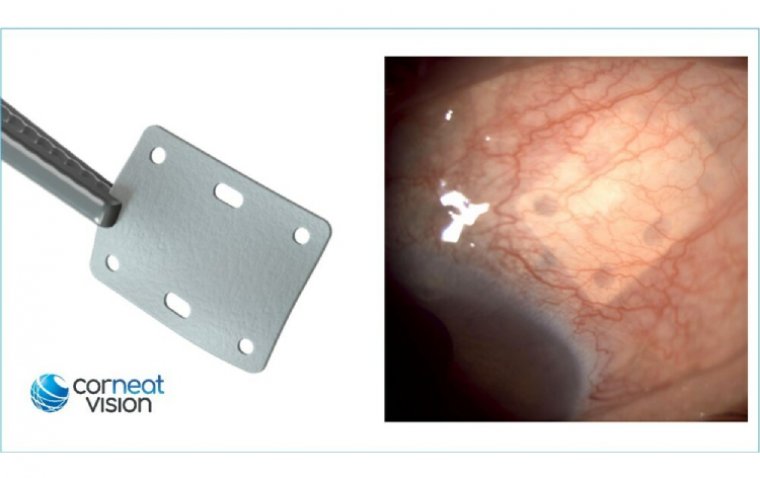
The CorNeat EverPatch serves as a synthetic tissue substitute for ocular surface surgeries, offering a distinctive blend of features that surpass existing tissue grafts prone to degradation over time. Its innovative structure facilitates seamless integration with surrounding tissue without eliciting a chronic foreign body response, while its non-degradable nature provides lasting reinforcement to the ocular surface.
The device is particularly adept at reinforcing tissue over glaucoma drainage devices, effectively mitigating complications like tube exposure that often necessitate reoperation. Remarkably thin at just one hundred microns, the CorNeat EverPatch boasts superior conformity to the eye wall compared to traditional tissue grafts.
Dr. Gilad Litvin, Co-Founder and Chief Medical Officer at CorNeat Vision, underscores the safety and efficacy of the CorNeat EverPatch, stating, "Our synthetic tissue substitute is tear-resistant, non-degradable, and completely bio-compatible, with no observed chronic inflammatory response that exists around tissue grafts. We are tracking over one hundred devices that we supplied to 25 US ophthalmic centers in the past couple of months, with excellent feedback. We are confident that the CorNeat EverPatch will set a new standard of care in ocular surgeries necessitating scleral reinforcement."
Positive Feedback from Surgeons
Distinguished surgeon Dr. Jason Bachrach, Medical Director at North Bay Eye Associates, lauds the CorNeat EverPatch based on his recent experience, commenting, "In a recent glaucoma surgery, I used the CorNeat EverPatch to cover an Ahmed valve and was delighted to experience how easy it was to handle and suture. It is significantly thinner than sclera or cornea, which is a notable advantage. Following the surgery, as well as two months post op, the patient's eye looked great. The fact the device is translucent leads to a truly aesthetic result."
Now available in the United States and select countries, CorNeat Vision eagerly anticipates showcasing the CorNeat EverPatch at the upcoming American Glaucoma Society (AGS) and American Society of Cataract and Refractive Surgery (ASCRS) Annual Meetings.
(1).jpg)