EyeCool Therapeutics Reports Positive Pilot Study Results for ETX-4143 in Chronic Ocular Surface Pain (COSP)
EyeCool Therapeutics has announced encouraging results from a double-masked, randomized controlled pilot study evaluating the safety and efficacy of its investigational device, ETX-4143, for the treatment of Chronic Ocular Surface Pain (COSP). The trial, conducted in 31 patients in Australia, showed a favorable safety profile and meaningful reductions in patient-reported pain scores.
Pilot Study Design and Key Findings
The pilot study (NCT06479382) was designed to evaluate ETX-4143 in a controlled, double-masked setting. While the trial was not powered for statistical significance, a statistically significant reduction in eye pain severity was observed using a recently validated patient-reported outcome (PRO) tool specifically tailored for COSP.
Topline results were first presented at the American European Congress of Ophthalmic Surgery (AECOS) Winter Symposium. Full study data are currently on file at EyeCool Therapeutics and will be submitted for peer-reviewed publication.
ETX-4143: A Targeted Device-Based Approach
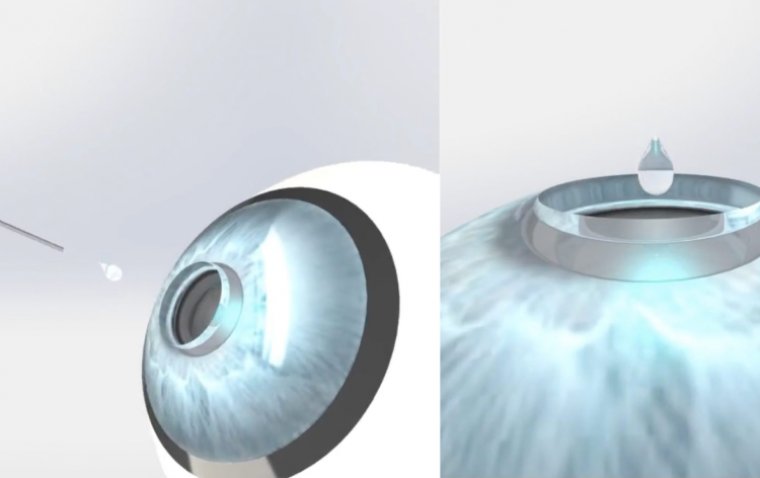
ETX-4143 is a non-invasive, investigational device developed for use during an in-office, outpatient procedure. It delivers gentle cooling to the surface of each eye over a 4-minute treatment period, aiming to modulate pain signals by targeting the myelinated long ciliary nerves.
Most patients reported immediate relief from ocular pain, with continued improvement over the following weeks. As nerve fibers gradually remyelinate over 2 to 3 months, symptoms may return, and repeat treatment may be required.
A Step Toward Addressing an Unmet Clinical Need
“This milestone reinforces the potential of our novel technology to address a critical unmet need for patients suffering from COSP,” said Dr. Ruben F. Salinas, President & CEO of EyeCool Therapeutics. He added that the company plans to initiate a large, U.S.-based pivotal trial following FDA Investigational Device Exemption (IDE) approval. The goal is to support a De Novo classification request upon trial completion.
The need for effective treatments for COSP was recently highlighted by expert physicians and patient advocates during the American Society of Cataract and Refractive Surgery (ASCRS) annual meeting.
Expert and Patient Advocacy Perspectives
“Chronic ocular surface pain is a common complaint that brings many patients to see an eye doctor. However, it is a condition that often goes undiagnosed or misdiagnosed as dry eye, leaving patients untreated,” said Dr. Preeya K. Gupta, Cornea and Cataract Surgeon at Triangle Eye Consultants in Raleigh, North Carolina. “Currently, we have no viable options to offer these patients, and having a solution such as the one being developed by EyeCool would be a very welcome addition.”
Rebecca Petris, co-founder and president of the Dry Eye Foundation, emphasized the burden of COSP on patient quality of life. “For most patients with dry eye, the biggest problem is persistent pain that affects their daily activities. These symptoms—burning, grittiness, light sensitivity, irritation—are all forms of pain. For better outcomes, we need industry and research to engage directly with COSP, and we’re pleased to see this sign of progress.”
(1).jpg)