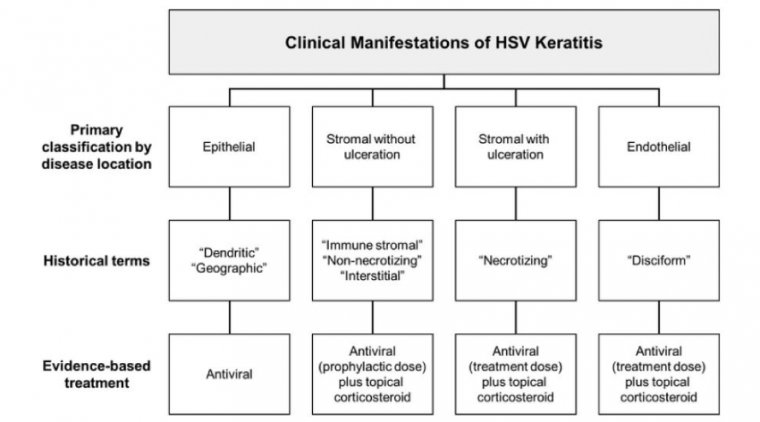
How to Deal with the Epithelial Herpetic Keratitis
Trifluridine eye drop, acyclovir (ACV) ointment, ganciclovir gel, and oral ACV are still the main therapeutic agents. Cryopreserved amniotic membrane has been recently used as an adjuvant treatment.
Resistance to ACV has become a concerning issue. The animal models of HSV vaccine are able to reduce HSV keratitis. New antivirals are under development.
Developing a Treatment Algorithm
Ocular infection with herpes simplex virus (HSV) affects approximately 400,000 individuals in the US and is a leading infectious cause of corneal blindness in the developed world.
Of the various presentations of HSV eye disease, herpetic keratitis (HK) is the most common and, in its most severe form, is a major indication for corneal transplantation. Approximately 20,000 new cases and 48,000 recurrences of HK are diagnosed each year in the US, although other estimates suggest a higher incidence.
A relatively uncommon condition, comprehensive ophthalmologists and optometrists may see only a handful of HK patients each year. Nonetheless, its recognition and management are critical due to its potential to progress and cause corneal scarring that can permanently compromise eyesight.
Accurate diagnosis and appropriate treatment can lead to better outcomes. Optimal management of epithelial (also called “dendritic”) HK starts with establishing a diagnosis as soon as possible.
Accurate characterization of disease extent prevents the misuse of potentially harmful treatments, such as corticosteroids, when they are not appropriate.
Once the diagnosis of dendritic HK has been made, the goal of antiviral therapy is to provide effective and efficient viral inhibition at the site of infection, with minimal ocular or systemic toxicity, in a dosing form that is convenient and comfortable. Clinician surveys have shown considerable variability in the treatment of HK.
This committee members Marguerite B. McDonald, MD, Chair, is a cornea specialist with Ophthalmic Consultants of Long Island, Lynbrook, NY.
She is a clinical professor of ophthalmology at the New York University Langone Medical Center, New York, NY, and an adjunct clinical professor of ophthalmology at the Tulane University Health Sciences Center, New Orleans, LA.
David R. Hardten, MD, is a founding partner and director of clinical research at Minnesota Eye Consultants, Minneapolis, MN.
He is an adjunct associate professor of ophthalmology at the University of Minnesota Medical School, and an adjunct professor at the Illinois College of Optometry, Chicago, IL.
Francis S. Mah, MD, is director of the cornea and external disease service and co-director of the refractive surgery service at the Scripps Clinic, La Jolla, CA.
He is a consultant to the Charles T. Campbell Eye Microbiology Laboratory of the University of Pittsburgh Medical Center.
Terrence P. O’Brien, MD, is Charlotte Breyer Rodgers Distinguished Chair in Ophthalmology and co-director of ocular microbiology at Bascom Palmer Eye Institute of the University of Miami School of Medicine, Palm Beach, FL. Christopher J. Rapuano, MD, is director of the cornea service and co-director of refractive surgery at the Wills Eye Institute, Philadelphia, PA, and a professor of ophthalmology at Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA. algorithm for the diagnosis and pharmaceutical treatment of epithelial HK (dendritic ulcers) was developed to address discrepancies in clinical practice and move the medical and patient communities closer to their goal of reduced HSV-related ocular morbidity.
Herpes Simplex HSV types 1 and 2, the viruses responsible for dendritic HK, are among the eight members of the herpes family known to infect humans. Other viruses in the herpes family associated with ocular morbidity include varicella zoster virus (VZV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV).
Typically, ocular herpetic infection is life-long, with the initial infection followed by periods of latency. HSV ocular disease—whether a primary infection or a secondary reactivation—can take the form of blepharitis, conjunctivitis, epithelial keratitis, stromal or endothelial keratitis, or uveitis.
HSV is globally endemic. Historical references extend as far back as Hippocrates, whose descriptions of spreading skin lesions gave us the Latinized herpes from the Greek herpein, “to crawl.”
Humans are the only natural reservoir of HSV. HSV-1 is most associated with lesions in the facial area, and HSV-2 with genital outbreaks, although an increasing proportion of genital infections are caused by HSV-1.
The initial infection typically results from direct or indirect contact with lesions, salivary droplets, or genital secretions of a virus-shedding carrier. In the clinic, careful hand-washing and swabbing instruments with isopropyl alcohol or sodium hypochlorite can prevent inadvertent transmission to uninfected persons.
Following initial infection, HSV-1 establishes lifelong latency in the trigeminal ganglion near the ventral area of the brainstem. Latent virus has the potential to reactivate and travel back down the the trigeminal nerve to cause an ocular recurrence.
Changing Epidemiology
HSV-1 was once almost universally acquired in infancy. That appears to still be true in developing nations, but epidemiological studies suggest that primary acquisition of HSV-1 is becoming progressively delayed in industrialized countries.
German researchers identifying HSV-1 in the trigeminal ganglia of cadavers found it in just 18% of those under 20 years of age, but in up to 100% of those 60 or older.
Epidemiological studies in Minnesota suggest that approximately 400,000 Americans have developed ocular HSV disease, with about 20,000 new cases and 48,000 recurrences diagnosed each year.
More than one ocular site may be involved; breakdown of the Minnesota data suggests that approximately 72% of ocular HSV disease involves the corneal epithelium; 41%, the lid or conjunctiva; 12%, the corneal stroma; and 9%, the iris and associated uveal tract.
A retrospective study at the Cullen Eye Institute found recurrence rates in children to be similar to those in adults, although there was a higher risk of bilateral involvement among children (26%).
Corneal transplantation is the most common form of tissue transplantation in the US and carries a high rate of success. However, complications due to recurrent and newly acquired HSV infection may occur.
Among corneal transplant recipients with ocular HSV, between 2% and 7% experience HSV reactivation and recurrent disease.
Further, transmission of HSV by corneal transplant to a previously uninfected recipient is a rare but dreaded cause of primary graft failure.
Reactivation and Ocular Involvement
Primary HSV infections are almost never recognized as such at the time they occur.
The vast majority of patients with ocular HSV infections, approximately 95% to 99% of cases, present with either the first ocular occurrence secondary to a nonocular primary infection at a different site or as a recurrent ocular infection, with or without associated “cold sores” around the mouth or nose.
Although latent infection is near ubiquitous in the adult population, less than a third of immunocompetent adults experience clinical disease, and a still smaller subset is prone to frequent recurrence.
HSV-1 strains clearly vary in their predisposition to breaking latency. Studies have likewise revealed human gene variations that predispose individuals to oral lesion recurrences.
In addition, research has identified a variety of reactivation triggers, including psychological stress, illness, fatigue, menstruation, trauma, immunosuppression, UV-B exposure, and hypokinesia.
While patients may report psychological stress as a predictor of recurrence, the Herpetic Eye Disease Study (HEDS) failed to corroborate this association and attributed it to recall bias.
Of particular importance to eye physicians is the realization that intense light exposure can trigger recurrence of herpetic ocular disease and may partially explain its postoperative incidence after LASIK and PRK procedures.
While reported less, the local trauma of invasive procedures such as cataract removal and lamellar keratoplasty likewise carry the risk of this complication.
Risk appears to be highest for patients who have previously experienced ocular herpes. However, patients with no history of ocular HSV can also present with this complication following ophthalmic surgery, and a patient history of frequent labial or nasal herpes may indicate a general predisposition to reactivation.
It is unclear whether topical ocular steroids can trigger reactivations of herpes virus in humans. According to studies performed in animals, ocular steroids themselves do not appear to trigger reactivation.
However, reactivation in the presence of steroids may be associated with unusually aggressive disease.
Primary vs Recurrent Disease
HSV can cause an array of ocular disease states. Primary ocular infections typically involve rapidly spreading viral dendrites or geographic ulcers in the corneal epithelium, because there is no antibody in the tear film to act against the virus.
In these cases there is no associated immune reaction to cloud the stroma or deeper ocular tissues. By contrast, recurrent infection can unfold against the background of a pre-primed immune response.
In many cases, the recurrent disease remains confined to the epithelium in the form of infectious dendrites or geographic ulcers. In other cases, however, the immune reaction causes stromal edema, with invasion by lymphocytes, macrophages, and other white blood cells.
This stromal immune reaction, which is often associated with corneal scarring, poses the greatest lasting threat to vision.
Studies suggest that 10% of patients with epithelial keratitis will go on to experience stromal disease within a year, and patients with a history of multiple recurrences remain at increased risk for future recurrences.
Natural History of Epithelial HK
In some cases, dendritic keratitis may progress to form geographic lesions, marginal keratitis, and/or trophic ulcers.
Cases that involve deeper ocular tissues, including the corneal stroma and endothelium, require distinct evaluative and therapeutic measures that are outside of the scope of this monograph.
Diagnosis In nearly all cases, the diagnosis of epithelial HK is made entirely on clinical grounds with - out the need for viral diagnostic testing. Even in academic centers HSV culture and advanced methods of viral detection are infrequently attempted, with such methods typically limited to complicated or atypical cases.
Patient history and examination and, most importantly, the finding of an epithelial defect with the classic dendritic appearance on slit lamp examination, are typically sufficient to make the diagnosis.
Most randomized, controlled trials that have evaluated antiviral therapy have likewise depended solely on clinical criteria for the HK diagnosis.
Clinical Presentation
Patients with HK typically present with the classic signs and symptoms of ocular infection, including unilateral tearing, photophobia, gritty or “foreign body” sensation, and/or visual changes.
Patients may report ocular discomfort or pain. However, those with repeated recurrences may have reduced or absent corneal sensation due to damage to the terminal branches of the trigeminal nerve. Such patients may complain of mild discomfort, but little or no pain.
In the experience of the panel, intraocular pressure (IOP) may be elevated, particularly if associated with comorbid iritis. Although unilateral in the great majority of cases, studies have found ocular HSV in both eyes in between 1% and 12% of cases, depending upon study criteria.
Bilateral or prolonged HK suggests the presence of a comorbid condition such as atopy, immunodeficiency, or immuno - suppression related to transplantation.
Clinical History
Because nonocular primary HSV infections are almost never recognized as such, most patients are diagnosed with HK at the time of their first or a recurrent ocular infection.
In the experience of the panel, patients presenting with a first ocular occurrence are typically young adults, teenagers, or children. Patients with reactivation of latent ocular HSV infection may be able to recall prior ocular outbreaks characterized by similar symptoms.
In contrast, patients with primary ocular HSV infection or first ocular occurrence of a nonocular primary infection (eg, orolabial HSV infection) often have an unremarkable ocular and past medical history.
Patients with reactivation of latent HSV may report antecedent ocular trauma or intense UV light exposure.
Clinical Appearance
Most patients with their first episode of ocular HSV present with isolated keratitis. However concomitant infection of adjacent tissues may be evident, therefore a careful examination of the periocular skin, regional lymph nodes, and conjunctiva is appropriate.
Examination of patients with primary infection or first ocular infection may reveal active or recently healed HSV dermatoblepharitis, blepharoconjunctivitis, or, rarely, conjunctivitis alone.
Dermatologic involvement—in the form of grouped vesicular or vesiculopustular eruption on the eyelid and adjacent skin—is common among patients with primary infection. Preauricular lymphadenopathy may also be present.
Isolated dermatoblepharitis or conjunctivitis may resolve spontaneously, or may progress to keratitis, typically within 7 to 10 days. HSV conjunctivitis can produce a follicular reaction indistinguishable from mild forms of adenovirus conjunctivitis.
However, unlike adenovirus, HSV conjunctivitis rarely forms pseudomembranes. The presence of dendrites on the conjunctiva— although not “classic” in appearance—can help confirm this diagnosis.
With recurrence, conjunctivitis may occur without eyelid or corneal involvement; therefore HSV should be included in the differential diagnosis when patients present with follicular conjunctivitis alone.
Corneal Staining
Topical instillation of water-soluble stains, such as fluorescein, rose bengal, and lissamine green B, aid in visualization of corneal and conjunctival defects and may be useful in the diagnosis of HK.
Each of these agents has a unique chemical structure and set of properties that enables it to highlight distinct pathological features.
Fluorescein is an orange dye that, when taken up by damaged epithelial cells and viewed under blue light, emits a bright green fluorescence. Fluorescein is used primarily to aid in the diagnosis of erosions, corneal abrasion, and keratitis.
It may be applied to the cornea using fluorescein-impregnated filter paper or via 0.25% solution. Rose bengal solution may be used in the evaluation of dendritic herpetic keratitis, superficial punctate keratitis, and other conditions.
Rose bengal stains damaged epithelial cells at the margins of HSV-induced dendritic ulcers bright red, but stains the ulcer base poorly. Recent research has shown that rose bengal has a cytotoxic effect on animal and human corneal cells.
It has also been shown to inhibit growth of protozoa, bacteria, and viruses. For this reason, tissue specimens for viral cultures or PCR should be taken in advance of diagnostic staining with rose bengal.
Some advocate the use of topical anesthetics before rose bengal to prevent the ocular irritation associated with this dye; others argue that this may contribute to false positive staining results.
Lissamine green is a synthetic dye with a staining profile similar to that of rose bengal. Lissamine green stain is more easily seen over the white sclera than the black pupil so, like rose bengal, it is more helpful in visualizing conjunctival than corneal tissue.
Lissamine green, however, has not demonstrated cytotoxicity to human cells and may be better tolerated by patients. Unlike rose bengal, lissamine green has not been shown to inhibit viral growth in vivo, although for reasons that are not known it may interfere with HSV detection by PCR.
Viral contact with materials present in collection swabs may also contribute to inaccurate testing results. In order to minimize false negative findings, it is recommended that clinical specimens being tested for HSV by PCR be collected before staining with either rose bengal or lissamine green and that sampling be performed with a cotton-tipped swab rather than a calcium alginate swab.
Corneal Appearance
Early in its course, prior to formation of a dendritic ulcer, HK may appear as small, raised corneal vesicles. Application of fluorescein stain aids in the visualization of the vesicles by pooling around their edges.
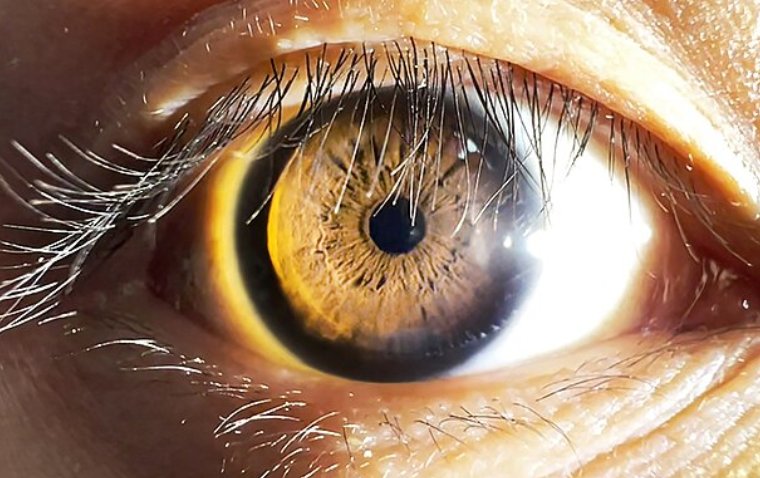
Corneal vesicles are the ocular corollary to vesicles that appear on skin and mucous membranes in early dermatologic HSV eruptions. Punctate or linear epithelial ulceration may also precede the formation of a classic dendritic lesion (Figure 3).
Prior to the formation of classic dendrites, thin branching lesions may give the appearance of pseudodendrites of VZV infection, which lack central ulceration or terminal bulbs.
In time, predendritic HSV lesions coalesce to form the classic dendritic appearance of HK, characterized by a branching shape and bulbous termini. Punctate satellite lesions or stellate lesions may also be observed.
Fluorescein staining reveals damaged corneal epithelial cells at the ulcer base and edges.1 Rose bengal and lissamine green lightly stain the base, and help demonstrate the raised edges surrounding the ulcer that contain active HSV.
A typical appearance of dendritic ulceration on slit lamp examination provides evidence of HK sufficient to warrant treatment. Laboratory evaluation Herpetic keratitis is a clinical diagnosis.
Under most circumstances, the observation of classic dendritic keratitis serves as the basis for initiation of treatment with topical antiviral therapy.
However, multiple laboratory techniques are available to assist diagnosis under rare circumstances, such as complicated cases, neonatal cases, or cases in which a definitive diagnosis is necessary.
Improved technology for rapid, reliable HSV diagnosis is currently under investigation. Direct Visualization Corneal specimens taken from the edge of the ulcer may be directly examined for evidence of HSV infection.
Light microscopy may reveal the presence of multinucleated giant cells on Giemsa stain, and intranuclear (Cowdry type A) inclusions on Papanicolaou stain.
Electron microscopy may reveal the presence of HSV particles in the nuclei of epithelial cells, and enveloped or mature viral particles in the cytoplasm.
Virus Culture HSV may be recovered from untreated dendritic ulcers by swabbing the ulcer with a softtipped applicator and inoculating it into 2.0 mL of viral transport medium or placed in a viral culturette.
Current options for viral detection include cell culture, an enzyme-linked virus-inducible system (ELVIS®), and polymerase chain reaction (PCR). Culture in cellular medium was traditionally considered the gold standard for HSV detection, since it indicates the presence or absence of active infection.
However, false negative results are common, particularly among patients exposed to topical antiviral treatment or whose eyes have been stained with rose bengal or lissamine green.
False negative results may also occur among untreated patients without such exposures, which may be a result of the body’s natural immune reaction to the infection or other factors that affect viral transfer and growth in vitro.
When positive, cell cultures show zones of virus-induced cytopathology within 1 to 3 days of inoculation, although it may take up to 1 to 2 weeks on rare occasions.
Due to potentially lengthy turnaround time, standard HSV culture is not useful for rapid clinical diagnosis. The ELVIS® HSV ID/Typing system is an accelerated version of standard virus culture, producing results within 24 hours of medium inoculation.
It uses a specially engineered cell line that, when infected with HSV, induces the expression of the enzyme beta-galactosidase, which is detectible by staining.
Compared with various other methods of cell culture, ELVIS® has demonstrated a high degree of sensitivity and specificity of clinical HSV detection. DNA Detection PCR may be used to detect the presence of HSV DNA in clinical specimens that contain live or inactive virus.
HSV PCR has been shown to be more sensitive than standard or accelerated cell culture.15,21 PCR may be used to establish the diagnosis in the face of a negative HSV culture, or as the primary definitive laboratory modality.
PCR results are typically available within 24 to 48 hours when performed in an in-house facility, or within 3 to 5 days when an outside facility is used.
Differential diagnosis In the experience of the committee members, the differential diagnosis of HK includes healing abrasion, drug-related effects, and corneal infection caused by Acanthamoeba, fungal, bacterial, or other viral pathogens.
The condition most clinically akin to HK, and the one with which it may be most easily confused, is VZV keratitis, which is associated with herpes zoster ophthalmicus (HZO).
VZV Keratitis VZV is the etiologic agent of both varicella (chickenpox) and its reactivation state herpes zoster or, more colloquially, “shingles.” Like HSV, VZV is a herpes virus that can establish latency in the trigeminal ganglion and reactivate along the ophthalmic branch of the trigeminal nerve to cause infectious keratitis.
Unlike HSV, reactivation of VZV typically happens only once in life in approximately 30% of adults.
Prior to the introduction and widespread implementation of the varicella vaccine in 1995, nearly all children acquired infection with the highly contagious virus and developed chickenpox, a self-limited disease characterized by fever, malaise, and a diffuse vesicular rash.
Following recovery from primary infection, VZV establishes latency in sensory root ganglia, which is maintained by a strong T-cell-mediated immune response. In most individuals, VZV-specific immunity after natural infection is life-long, and the virus remains latent.
However, in approximately 20% to 30% of individuals, latent VZV reactivates along one or more sensory dermatomes, causing shingles (related, it is thought, to waning T-cell immunity with advancing age).25 Unlike HSV, herpes zoster is characteristically more common and more severe with advancing age and among immunocompromised individuals.
The varicella vaccine program has markedly altered the epidemiology of not only varicella, but also shingles. The incidence of shingles is increasing as the proportion of elderly individuals in the population expands; and it is also increasing among adults in the 40 to 50 year old age range.
This is thought to be a result of the near disappearance of childhood chickenpox since the vaccine was introduced—exposure to cases of chickenpox may have served as a physiologic “booster vaccine” to exposed mid-life adults.
HZO results from VZV reactivation in the trigeminal ganglia and travels through the ophthalmic division of the fifth cranial nerve. HZO occurs in approximately 10% to 20% of individuals with shingles, making it the second most common anatomical site of VZV reactivation after the torso.
HZO is typically associated with a painful, unilateral rash extending above and/or below the eye along the sensory dermatome. Over the course of several days to weeks, dermatologic lesions may evolve from maculopapular to vesiculopustular to crusted.
Approximately 50% of HZO cases that do not get treatment within the first 72 hours of the appearance of the rash will develop ocular involvement. Corneal complications include punctate or pseudodendritic epithelial keratitis, stromal infiltrate, endotheliitis, and neurotrophic keratitis.
HZO may be associated with significant morbidity including visual loss. Because their pathophysiology, treatment, and prognosis are different, differentiating HSV from VZV ocular disease is important.
In the clinical experience of the panel, ocular HSV tends to present in young to middle-aged individuals, whereas VZV is generally seen in older patients. Distinguishing characteristics of HZO include a prodrome of fever, malaise, headache, or pain or tingling along the forehead or scalp; HZO may be accompanied by changes in affect including moodiness, depression, and insomnia.
The HZO rash is frequently associated with exquisite pain, and distribution along a sensory dermatome is unique to VZV. HZO affects the deep dermis, may cause periorbital edema and ptosis, and can result in permanent scarring.
By contrast HSV only affects the epidermis.26 Although rare among patients with HZO, VZV (like HSV) can cause significant keratitis in the absence of skin involvement.
In such cases, a key to differentiation is the appearance of the fluorescein-stained corneal lesions.
Both agents may cause punctate epithelial keratitis early in the infection, but in contrast to the true dendrites associated with HSV, VZV keratitis is characterized by pseudodendrites, which stain less intensely, are elevated, and appear as tapering lines without central ulceration or terminal bulbs.
Other Conditions
In addition to VZV, the differential diagnosis of HSV keratitis includes infections caused by EBV, CMV, Acanthamoeba, and a variety of bacterial and fungal pathogens. Ocular complications of CMV and EBV infection are not encountered with great frequency, even among cornea specialists.
Like HSV and VZV, CMV and EBV are members of the herpes virus family, and both are globally endemic. In contrast to HSV, which persists in neuroganglia, CMV and EBV establish latency in white blood cells such as T cells and monocytes.
Ocular disease related to CMV most commonly occurs among immunodeficient individuals.27 However, CMV keratitis, predominantly endotheliitis, has been reported in immunocompetent persons as well.
Systemic EBV infection may be associated with a wide range of ocular manifestations including epithelial keratitis. Acanthamoeba keratitis (AK) is a rare, severe, protozoan infection that may be mistaken for HK due to a similar dendritic pattern on the corneal epithelium early in the course of the disease.
In the clinical experience of the committee, Acanthamoeba-induced lesions appear elevated and lack the terminal bulbs which help to distinguish HK lesions. Patients with AK commonly have a history of contact lens wear or trauma, and may complain of severe pain that seems disproportionate to physical findings.
Non-infectious processes that may be mistaken for HSV keratitis include healing abrasions and drug-related toxicity. Healing corneal abrasions may have a dendritiform appearance. However, they do not demonstrate a classic, “tree-branching” pattern and lack terminal bulbs.
Patients on topical ophthalmic medication for the treatment of HSV infection or other conditions may develop keratitis medicamentosa which may be misdiagnosed as progressive or intercurrent HSV infection.
Medicamentosa is a toxic reaction to topical ophthalmic medication or a combination of medications and is characterized by redness and/or erosions of the conjunctiva and cornea that may range from mild to severe.
When an allergic component is present, patients may also experience itching, swelling and redness of periorbital tissue; eosinophils may be present in affected tissue. Nonselective antivirals, such as trifluridine, and cytotoxic antibacterials, such as aminoglycosides, are common culprits.
Topics in Treatment An evolving standard of care Without treatment, most cases of superficial dendritic keratitis will resolve without permanently damaging vision.
But with no way of predicting which infections will progress, early and effective treatment is imperative to minimize the risk of progression.
Treating the disease also allows for relief of associated symptoms such as pain, irritation, redness, and discharge.
Management Prior to Antiviral Availability
Prior to the development of antiviral therapies, HK was treated by physical debridement of eroded tissue and application of iodine, a painful and difficult procedure that was often ineffective.
Sometimes it was necessary to perform a conjunctival flap, a procedure that relieved pain, but functionally blinded the patient.
Non-selective Topical Antiviral Agents
The development of idoxuridine (IDU), the first effective antiviral medication for any organ, revolutionized the field of infectious disease and ushered in a new era in the treatment of ophthalmic viral infections.
IDU was a nucleic acid analog originally under investigation as an anticancer drug. It worked by binding and blocking DNA polymerase; in effect, it tricked the herpes virus into taking it up as if it were a normal nucleic acid and thus committing suicide.
Limiting IDU’s effectiveness against ocular herpes was the drug’s lack of solubility, which required that it be applied every 2 hours around the clock. Another nucleoside analog, vidarabine ophthalmic ointment 3% (also known as adenine arabinoside, ara-A, or vira-A) was approved by the US FDA in 1976 for the treatment of acute keratoconjunctivitis and recurrent epithelial keratitis due to HSV.
Vidarabine was more effective than IDU and required less frequent dosing.2 With the evolution of ophthalmic antiviral agents toward more selectivity and less toxicity, vira-A ophthalmic ointment use diminished markedly, and it is rarely used today.
While difficult to find, it may be compounded at some specialty pharmacies and may be useful in patients recalcitrant to, or intolerant of, other antiviral agents.
Like IDU, trifluridine (also known as trifluorothymidine or TFT) was a substituted nucleotide originally used in oncology to block the mass assembly of nucleic acids in rapidly reproducing cancer cells.
Trifluridine proved to be an effective ocular antiviral with high success rates in the treatment of HK infections and demonstrated superiority to IDU and vira-A in clinical trials.3 By the 1970s, trifluridine had become a drug of choice in the treatment of ocular herpes.
Non-selective antiviral agents inhibit not only virus reproduction but also cellular DNA synthesis in uninfected cells. As such, non-selective antiviral agents can interfere with wound healing and contribute to ocular surface toxicity.
Associated complications are rare, but can be serious when viral infection produces large corneal ulcers that require significant cell division to heal. Some non-selective antivirals are preserved with thimerosal, a mercury-based preservative.
Like long-term topical aminoglycoside use, prolonged use of such agents is a recognized cause of monocular blepharitis. Other serious side effects include allergic reaction and the occlusion of lacrimal drainage.
Selective Oral Antiviral Agents
The development of acyclovir, the first selective nucleoside analog, marked the next major advance in the fight against herpes viruses.
The mechanism of action of acyclovir was novel in that it exploited slight differences in viral and cellular versions of thymidine kinase (TK), the enzyme that activates the precursor form of the drug into its active form.
As a substrate for viral TK only—it is not acted upon by the TK found in human cells—acyclovir ushered in a new generation of antivirals with potency against HSV-infected cells and lower toxicity potential.
As the first virus-selective agent, acyclovir was also the first antiviral appropriate for systemic use. HEDS addressed the question of whether or not long-term systemic antiviral therapy could prevent ocular HSV recurrences and found that oral acyclovir could reduce HK recurrence rates by approximately 40% to 50% overall.
Unfortunately, HEDS also showed that oral antiviral therapy could not prevent progression from surface infection to deeper stromal disease. Acyclovir available for oral administration is indicated for the acute treatment of shingles, genital herpes, and chickenpox.
Other viral enzyme inhibitors have been developed that share acyclovir’s mechanism of action and selectivity for virus-infected cells. Valacyclovir is a valine ester of acyclovir, and has the advantage of being much more bioavailable when taken orally.
In contrast to acyclovir which is poorly absorbed, valacyclovir passes readily into the bloodstream, where it is metabolized into its active form, acyclovir.
Famciclovir is an acyclic guanine derivative with a longer intracellular half-life than acyclovir. Neither valacyclovir nor famciclovir has been compared for efficacy to acyclovir in large, controlled clinical trials, but is presumed to be similar.
Selective Topical Antiviral Agent
Ganciclovir ophthalmic gel (marketed outside the US as Virgan®) was approved for the treatment of acute HK in France in 1995 and has since been approved in 30 other countries outside of the US.
Approved by the US FDA in 2009, ganciclovir ophthalmic gel 0.15% (Zirgan ®) became the first new topical drug for herpetic eye disease in the US since the advent of trifluridine in the 1970s.
In intravenous and ocular implant formulations, ganciclovir was already well known to ophthalmologists as an effective treatment for CMV-related eye disease, including some cases that were unresponsive to acyclovir.
Like acyclovir, ganciclovir specifically targets HSV DNA in infected cells, making it the first (and only) selective topical antiviral agent available in the US for the treatment of dendritic HK.
The mechanism of action of ganciclovir starts with a series of phosphorylations that converts it into active form. This activation takes place preferentially inside cells infected by HSV and is the basis of its specificity for virus-infected cells.
Once activated, ganciclovir’s mechanism of action is two-fold: it slows viral replication by competitive inhibition of viral DNA polymerase; and it directly incorporates into the viral DNA primer strand. This terminates the viral DNA chain and shuts down virus replication.
Ganciclovir ophthalmic gel clinical trials were conducted at sites outside of the US where the antiviral comparator agent of choice, acyclo - vir ophthalmic ointment 3%, was commercially available. In Europe, the safety and efficacy of acyclovir ophthalmic ointment is well estab - lished.
Both acyclovir and ganciclovir selec - tively target virus-infected cells and are better tolerated than first-generation agents, including trifluridine.
Thus, although never marketed in the US, acyclovir represented the most rigorous standard of comparison for ganciclovir. These trials showed that ganciclovir gel was as effective as acyclovir ointment in treat - ing dendritic HK, with significantly higher patient tolerability.
Three-quarters of patients in these studies rated the tolerability of ganciclo - vir gel as “excellent,” and 97% rated it “good” or “excellent.” Ganciclovir gel is preserved with a low con - centration of benzalkonium chloride (BAK).
BAK has been shown to be gentler on the conjunctiva and cornea compared with thimerosal. Thimerosal has been associated with marked cytotoxicity to human corneal cells in vitro, and sensitization and significant allergic reaction in patients.
Tolerability of ganciclovir gel has been excellent in clinical trials. Ganciclovir gel may be stored at 59° F – 77° F (15°C – 25°C), eliminating the need for refrigeration. Refrigeration before dispensing can limit pharmacy availability.
One drop of ganciclovir gel should be instilled in the affected eye five times daily (every 3 hours while awake) until the corneal ulceration heals, then three times daily for the subsequent week.
Oral Antiviral Monotherapy
There is a dearth of data on the use of oral antiviral therapy as a substitute for topical anti - viral therapy in the treatment of acute HK. This is understandable, as topical treatment is a well accepted form of pharmaceutical administration for ophthalmologic conditions.
A randomized, double-blind, controlled trial found that treatment of HK with oral acyclovir was associated with similar efficacy and speed of healing compared with topical acyclovir, suggesting that oral therapy was a reasonable alternative to topical among patients who might not tolerate the topical.
Oral Antiviral as Adjunct to Topical Therapy
Some clinicians report the use of oral an - tiviral therapy as an adjunct to topical antiviral therapy in the treatment of acute HK.
A meta-analysis of HK therapies revealed that combination oral and topical antiviral therapy had similar efficacy compared to topical antiviral monotherapy (relative risk [RR]=1.08; 95% confidence interval [CI] 0.99 to 1.17).
Authors concluded that it remains unclear whether the addition of a second antiviral agent to a baseline topical antiviral regimen is useful in accelerating healing. Further studies are needed to assess the role of adjunctive oral antiviral therapy in the treatment for dendritic epithelial keratitis.
Oral Antiviral to Address Recurrence
As a recurrent disease with significant and potentially cumulative morbidity, the ability to prevent outbreaks of ocular HSV is attractive to clinicians and patients.
Systemic antivirals for the suppression of recurrence may play a role in the management of ocular HSV disease in select patients.
The HEDS found that oral acyclovir (400 mg, twice daily) for 1 year significantly reduced the risk for recurrence of ocular HK, stromal keratitis, and orofacial HSV compared with placebo.
Follow up at 6 months after prophylaxis was stopped showed that the benefit was not sustained off medication, although the infection rate did not rebound.
Furthermore, the benefit provided by long-term acyclovir pro - phylaxis in the prevention of stromal keratitis was seen only in those with a history of stro - mal keratitis and was not observed among subjects with no history of stromal involvement.
In other words, oral prophylaxis did not seem to prevent progression from superficial HK to stromal disease.
According to a community-based retro - spective chart review conducted in Olmsted County, MN, long-term oral antiviral prophylaxis (mean: 2.8 years) markedly reduced rates of epithelial keratitis, stromal keratitis, blepharitis, and conjunctivitis compared with no prophy - laxis over a mean 7.7 years of follow-up.
These data are consistent with benefits noted in the HEDS and suggest that prophylaxis beyond 12 months may improve outcomes further. Most research regarding oral antiviral ther - apy of HSV infections centers on the use of acyclovir.
However more is becoming known about agents that offer superior pharmacokinetic properties and reduced dosing frequency. Valacyclovir 500 mg once daily was compared with acyclovir 400 mg twice daily in the prophylaxis of HSV ocular disease and shown to be similarly effective.
This pilot study found a 23% recurrence rate over the course of 1 year with either agent. Adverse events, the most common of which were nausea and headache, were similar in frequency, severity, and type between the two treatment arms.
At present, long-term prophylaxis with an oral antiviral agent for the prevention of HSV ocular recurrences is recommended for select populations only: patients with severe stromal keratitis, patients with frequently recurring epithelial keratitis (more often than one episode per year), and corneal transplant patients with history of ocular HSV.
Either oral acyclovir (400 mg twice daily) or valacyclovir (500 mg once daily) are suitable options in patients who main - tain good renal function.
Antiviral Resistance
HSV resistance to acyclovir and related antiviral compounds, valacyclovir, famciclovir, and penciclovir, has remained low since introduction of these agents starting in the early 1980s.
Resistance is far more common among immunocompromised patients—estimated 3.5% to 10% compared with 0.1% to 0.7% among immunocompetent patients—as viral replication is typically prolonged in such patients, and impaired host responses allow for virus with lower pathogenicity to survive and replicate.
In fact, infection with an HSV-1 strain that is resistant to acyclovir raises the concern of im - munodeficiency. The vast majority of HSV-1 strains with clinical resistance to acyclovir contain a mutation in the gene that encodes for the key enzyme TK.
While some TK-mutant strains remain highly pathogenic, others have reduced “viral fitness” as a result of the mutation compared with wild-type strains. Rarely, the mechanism of antiviral resistance relates to an altered DNA polymerase.
The low rates of acyclovir resis - tance despite decades of widespread use may be a result of compromised pathogenicity in HSV1 strains with mutant TK or DNA polymerase.
Though rates are low overall, increased an - tiviral prescribing may be driving up acyclovir resistance in patients with herpetic eye disease. A recent study of sequential corneal HSV-1 isolates from patients with recurrent HK revealed an acyclovir resistance rate of 6.4%, surprisingly high for an immunocompetent population.
All patients in this study with an acyclovir-resistant HSV strain had received treatment with acyclovir within the past year. Lack of clinical responsiveness to treatment—slow healing over several weeks or frequent recurrences—was associated with antiviral resistance.
Acyclovir-resistant HSV strains are resistant to acyclovir’s prodrug valacyclovir, and most are cross-resistant to famciclovir. Ganciclovir and acyclovir share similar structures and mechanisms of action, therefore cross-resistance is possible.
In Duan and coworkers’ study of corneal HSV isolates, 45% (5/11) of acyclovir-resistant strains were resistant to ganciclovir.34 Recovery of HSV and in vitro resistance assays and molecular characterization of isolates should be performed prior to switching therapy in patients who are refractory to initial therapy.
This may necessitate a referral to a corneal specialist with expertise in infectious disease management.
(1).jpg)