Genetically Diverse Mouse Models Uncover Eye Aging Patterns and Links to Brain Health
New research from The Jackson Laboratory (JAX) highlights the critical role of genetics in retinal aging, demonstrating how different genetic backgrounds influence the aging process in distinct ways. The study, published in Molecular Neurodegeneration, analyzed nine genetically diverse mouse strains, offering insights into age-related vision loss and potential connections between eye health and neurodegenerative diseases.
A More Accurate Model for Eye Aging Research
Historically, studies on retinal aging have relied on single-strain mouse models, limiting researchers’ ability to assess genetic variability in eye diseases.
"The challenge in studying age-related eye diseases is that aging is heterogeneous," said Gareth Howell, PhD, Professor and Diana Davis Spencer Foundation Chair for Glaucoma Research at JAX, who led the research. "Observing how aging occurs in one strain of mice might not be relevant to all mice—or humans. To overcome these limitations, we sought to understand how genetic context drives retinal aging."
By examining age-related genetic and molecular changes in young and old mice from nine strains, Howell and his team have created a comprehensive dataset, now publicly available to support aging and vision loss research. Their work also strengthens the potential of retinal imaging as a biomarker for neurological diseases.
Genetic and Protein Analyses Predict Eye Disease Risks
A key discovery of the study was the identification of two mouse strains that closely resemble human retinal diseases.
• Watkins Star Line B (WSB) strain exhibited characteristics of age-related macular degeneration (AMD) and retinitis pigmentosa, a rare inherited form of blindness.
• New Zealand Obese (NZO) strain, known for severe obesity and diabetes, developed diabetic retinopathy.
Gene and protein analysis in these strains predicted the onset of common age-related eye diseases, reinforcing the value of genetic screening in ophthalmic research.
"It was promising to see that the molecular data we generated predicted specific retinal cell abnormalities in these two strains," said Olivia Marola, PhD, postdoctoral associate and co-first author of the study.
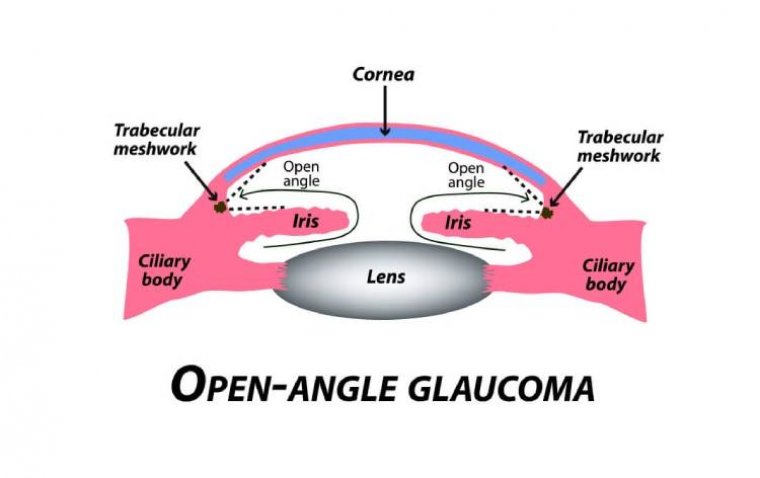
Further analysis revealed functional retinal changes at the cellular level, allowing researchers to track disease progression and explore potential treatments for conditions like cataracts, glaucoma, AMD, and diabetic retinopathy.
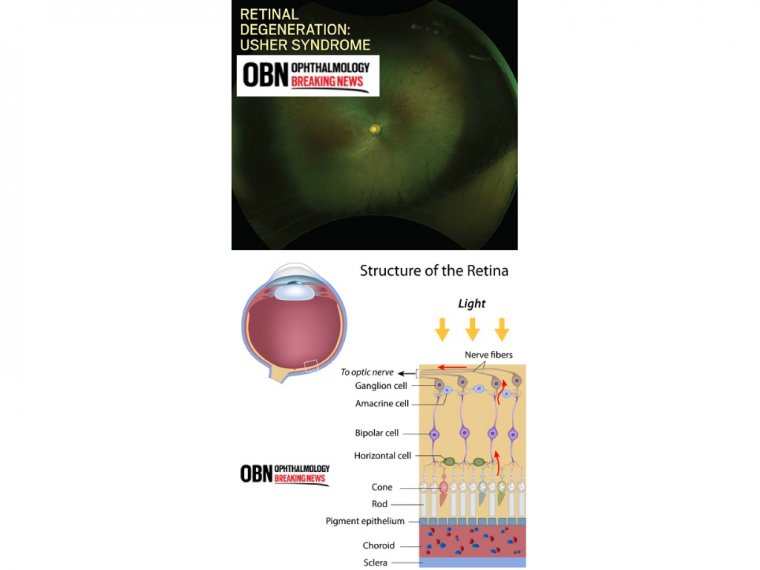
The Retina as a Biomarker for Alzheimer's Disease
Beyond ophthalmology, this study has broader implications for neurodegenerative diseases. Since the retina is an extension of the brain, understanding its aging process could provide new insights into conditions like Alzheimer’s disease and dementia.
"The eye is a crucial organ, and this research fills an important gap in our understanding of aging," said Howell. "By studying the aging retina, we may develop new ways to assess a person’s risk of developing diseases like Alzheimer’s."
Advancing Vision and Brain Health Research
The findings from this study enhance the understanding of eye aging and offer a new pathway for studying neurodegeneration. By leveraging genetically diverse models, researchers can now refine diagnostic and therapeutic approaches for both ocular diseases and brain disorders.
Reference:
Olivia J. Marola et al, Genetic context modulates aging and degeneration in the murine retina, Molecular Neurodegeneration (2025). DOI: 10.1186/s13024-025-00800-9
(1).jpg)