FDA Accepts Eluminex’s IND Application for Trispecific Fusion Antibody for DME
Eluminex Biosciences announced the acceptance of its EB-105 Investigational New Drug (IND) application by the FDA. This significant milestone marks a pivotal moment in the advancement of medical science, particularly in the realm of Diabetic Macular Edema (DME) treatment.
EB-105: A Trispecific Fusion Antibody
EB-105 stands as a groundbreaking trispecific fusion antibody, meticulously crafted to target a spectrum of crucial factors implicated in DME pathology. Specifically, it zeroes in on Vascular Endothelial Growth Factor A (VEGF-A) and its isomers, alongside VEGF-B, Placental Growth Factor (PlGF), Angiopoetin-2 (Ang-2), and the Interleukin-6 receptor (IL-6R).
In a recent statement, Dr. Quan Dong Nguyen, a distinguished authority in Ophthalmology, shared insights into the significance of this development. "Elevated levels of IL-6 in the vitreous have been well-documented in patients with DME, even with ongoing anti-VEGF treatments," Dr. Nguyen remarked. "The ability to inhibit several biological pathways simultaneously with a single agent offers compelling potential for further incremental benefits in patients with retinal diseases."
Beyond the realm of scientific discourse, Eluminex is actively seeking strategic alliances and investment partnerships to fortify its pioneering endeavors in both retinal disease and dermal facial aesthetics.
This acceptance of the EB-105 IND application by the FDA heralds a new chapter in the quest to combat DME and offers hope to countless individuals grappling with this debilitating condition. As research progresses and partnerships flourish, the potential for transformative impact in ophthalmic care becomes increasingly tangible.
About Diabetic Macular Edema
Diabetic Macular Edema (DME) represents a formidable challenge within the realm of ophthalmology, characterized by fluid accumulation in the macula due to compromised blood vessels in the retina. This condition arises as a complication of diabetic retinopathy, a microvascular complication of diabetes mellitus.
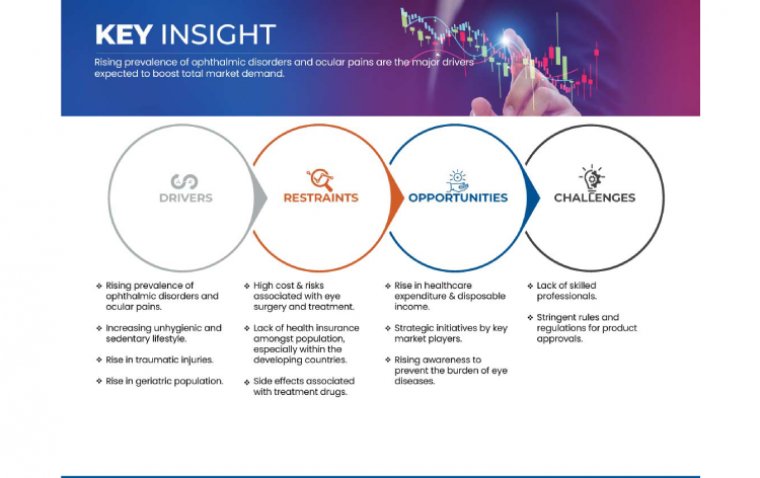
DME is a leading cause of vision impairment and blindness among working-age adults worldwide. Its pathogenesis involves a cascade of events triggered by chronic hyperglycemia, leading to vascular leakage, inflammation, and ultimately, macular edema. Despite advances in treatment modalities such as anti-vascular endothelial growth factor (VEGF) therapy, significant unmet needs persist, highlighting the pressing demand for innovative therapeutic approaches to alleviate the burden of this sight-threatening condition.
(1).jpg)