FDA Approves Cyclosporine Eye Drops For Vernal Keratoconjunctivitis In Adults And Children
Verkazia 0.1%) eye drops containing cyclosporine have been authorized by the US Food and Drug Administration (FDA) for the treatment of vernal keratoconjunctivitis (VKC) in both adult and juvenile populations.
The surface of the eyes in patients with the uncommon, recurrent allergic eye disease is severely inflamed. Intense scratching, sore eyes, and light sensitivity are possible symptoms.
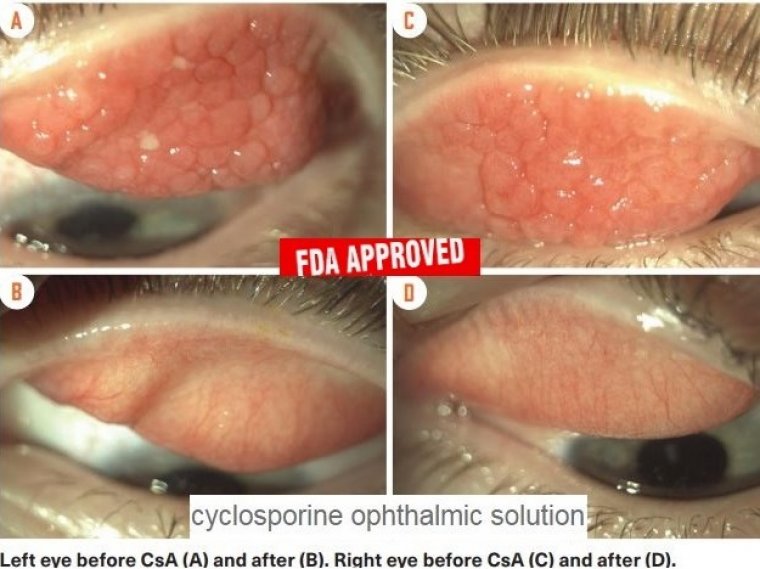
The indications and symptoms were immediately and dramatically decreased by cyclosporine A cationic emulsion (CsA CE), and the extended therapy with CsA CE stabilized patients while maintaining a good safety profile, according to the results of a 4-month effectiveness study1 and an 8-month safety follow-up period.
The FDA recently approved cyclosporine ophthalmic emulsion 0.1% (Verkazia; Santen Pharmaceutical Co, Ltd) for the treatment of vernal keratoconjunctivitis (VKC), a rare, recurrent allergic disorder.
Results of a 4-month efficacy study1 and an 8-month safety follow-up period2 showed that the signs and symptoms were quickly and significantly reduced by cyclosporine A cationic emulsion (CsA CE), and the extended therapy with CsA CE stabilized patients and did so while maintaining a good safety profile, according to Andrea Leonardi, MD, PhD, of the Department of Neuroscience, Ophthalmology Unit, University of Padua, Italy, and the senior author of both studies.
Most of the individuals affected are children, adolescents, and young adults who develop severe inflammation of the ocular surface. When the disease is severe, VKC negatively affects the patients’ quality of life, with symptoms including intense itching, ocular pain, and light sensitivity that can prevent those affected from participating in everyday activities.
The availability of this drug is important because without treatment, patients can progress to development of corneal ulcers and visual loss. Another plus associated with cyclosporine ophthalmic emulsion 0.1% is that it addresses the underlying pathology of VKC without the risk of long-term adverse ocular effects.
Cyclosporine ophthalmic emulsion 0.1% works by inhibiting T-cell activation and reduces the level of immune cells and mediators that cause the chronic, severe, potentially debilitating allergic inflammation of the ocular surface in VKC.
VEKTIS Study
The VEKTIS study (NCT01751126), a multicenter, double-masked, randomized controlled trial, assessed the safety and efficacy of cyclosporine A cationic emulsion 0.1% eye drops over a 12-month period in pediatric patients with severe active VKC.
Initially, 169 patients aged 4 to 17 years were randomized to the 4-month VEKTIS trial, and of those, 142 entered the 8-month follow-up period during which the patients randomly assigned to the cyclosporin A cationic emulsion stayed on their original treatment regimen, ie, drop instillation 4 times daily (high dose) or twice daily (low-dose plus the vehicle twice daily).
Those receiving the vehicle were later allocated to 1 of the 2 active regimens. The main outcome measures of the study were safety and efficacy. The investigators recorded adverse events (AEs) associated with treatment and corneal fluorescein staining score.
Compared with the patients assigned to vehicle, the patients on active treatment had improvements in staining scores, rescue medication use, and photophobia, tearing, itching, and mucous discharge.
The quality of life also improved, as assessed by questionnaire responses during the 4-month evaluation period. The patients remained stable during the 8-month follow-up period, according to the investigators, with the high-dose regimen continuing to provide greater benefits in most efficacy measures.
The drug was well tolerated; AEs related to treatment were found in 15 patients who received high-dose treatment and 11 who received low-dose treatment, and the AEs were related mostly to the instillation side.
Drug benefits “Results from the VEKTIS trial showed that the significant improvements in VKC symptoms and signs and the quality of life with topical CsA were maintained over 12 months, with a significant reduction in the use of topical corticosteroids,” Leonardi commented.
In addition, CsA CE provided a rapid onset of action in patients with severe VKC, ie, within the first month of treatment, and because it is corticosteroid sparing, the potential corticosteroids-associated AEs are avoided.
Patients reported a significant improvement of quality of life regarding their ability to participate in daily activities, subjective emotional well-being, and symptoms.
“CsA is a better drug for managing affected children, and parents express better ‘satisfaction’ with the drug considering that schooling, vacationing, and normal daily activities are compromised in affected children, which ultimately affects the entire family.
In addition, clinicians can better control the disease in the short and long term, prevent recurrences of the disease during the worst season, and can use [fewer] corticosteroids, which contributes to a safe profile of CsA,” Leonardi concluded.
Cyclosporine Eye Drops for Dogs
Cyclosporine eye drops are a prescription medication used to treat various eye conditions in dogs, such as dry eye, keratoconjunctivitis sicca (KCS), and immune-mediated keratitis.
Cyclosporine works by inhibiting the production of certain chemicals that contribute to inflammation in the eye. It also helps to increase tear production, which can be beneficial for dogs with dry eye.
Cyclosporine eye drops are usually administered twice daily, and it may take several weeks or months before the full effects of the medication are seen. It is important to follow your veterinarian's instructions and to give the medication as prescribed.
It is also important to note that cyclosporine eye drops may cause side effects in some dogs, such as increased tear production, eye irritation, and redness. If you notice any unusual changes in your dog's eyes or behavior, it is important to contact your veterinarian.
Overall, cyclosporine eye drops can be an effective treatment for certain eye conditions in dogs when used as directed. It is important to follow your veterinarian's instructions and to monitor your dog for any potential side effects.
(1).jpg)