The Process Of Unplugging The Clogged Drain In Glaucoma IOP
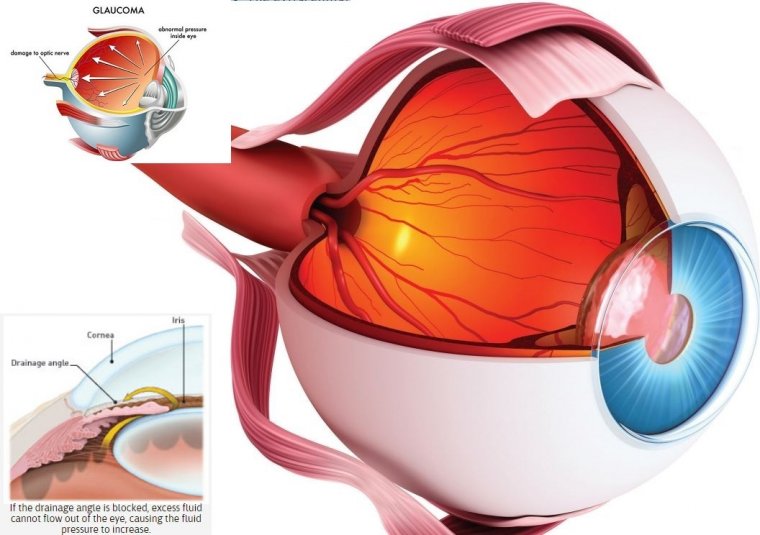
Glaucoma causes include elevated eye pressure (called intraocular pressure or IOP) due to the eye’s inability to drain fluid efficiently.
A clear fluid called aqueous humor circulates inside the front portion of our eyes. To maintain a constant healthy eye pressure, the eye continually produces a small amount of aqueous humor while an equal amount of this fluid flows out of the eye.
The fluid flows out through a very tiny drain called the trabecular meshwork, a complex network of cells and tissue in an area called the drainage angle.
In glaucoma, the aqueous humor does not flow through the trabecular meshwork properly. If the drainage angle is become less efficient at draining fluid, as in the common open-angle glaucoma, excess fluid cannot flow out of the eye properly, causing the intraocular pressure (IOP) to increase.
Over time, raised IOP causes damage to the nerve fibers. If the drainage angle becomes completely blocked, eye pressure rises quickly, resulting in a narrow-angle glaucoma or angle-closure glaucomaattack, with severe eye and brow pain, nausea and vomiting. This kind of glaucoma attack is a medical emergency and must be treated immediately.
How to Interrupt the Pathways & Disease Pathogenesis
Investigators are studying different ocular pathways to determine how to interrupt the pathways and disease pathogenesis.
With a better understanding about the outflow mechanisms in the eye, with the focus on the trabecular meshwork (TM), the drainage pathway, patients with glaucoma may not require as much follow-up as is needed currently.
TM Research
Glaucoma occurs at the TM and optic nerve. The juxtacanalicular tissue (JCT) region in the TM is the anatomic location that controls resistance. This area is not static and it is constantly undergoing remodeling.
The JCT region contains 2 main pathways: the paracellular pathway in between cells and the transcellular pathway through cells. The extracellular matrix, upon which the cells sit and their structure is maintained, is an important component in controlling the intraocular pressure (IOP), Rhee explained.
The increased amount of extracellular matrix in the JCT is the only known culprit in primary openangle glaucoma (POAG) that works by gumming up the flow of fluid through the JCT region.
Numerous medications are prescribed and numerous surgeries are performed to lower the IOP but they do not cut to the heart of the glaucoma process. Doctors are lowering IOP by altering the physiology but not by interrupting the disease pathophysiology.
And for this reason, glaucoma can still progress despite lowering of the IOP. Even though there may be no loss of visual fields, patients must be followed consistently over time to ensure adequate IOP control and treatment adjusted if control is lost.
A startling statistic is that 7% to 14% of patients become legally blind in 1 eye over a 2-year period despite the best of care because the root cause of the glaucoma is not being addressed by medication and surgery.
Transforming growth factor beta-2 (TGFß2) has been recognized as an important factor in the development of glaucoma.
The concentration of TGFß2 is elevated up to 3-fold in the aqueous humor of eyes with primary open-angle glaucoma and increases the IOP in perfused cadaveric human anterior segments as well as alterations in the JCT extracellular matrix resembling glaucoma.
TGFß2 also elevates the IOP in live mice and rats and causes optic nerve damage. Researchers hypothesize that anything that controls the extracellular matrix is relevant in IOP control. Matricellular proteins allow cells to control the surrounding extracellular matrix.
Doctors investigated a family of such proteins and found 3—SPARC and thrombospondin-1 and -2— that lower IOP in mice from which those genes have been removed.
A closer look at SPARC found that it is one of the most highly transcribed genes by the TM; it is found throughout the TM and highly concentrated in the JCT.
And importantly, SPARC is one of the most highly secreted proteins in response to TGFß2 and increases in IOP. It has been hypothesized that increased IOP was dependent on SPARC.
The most important finding was that if TGFß2 is administered to normal mice, the IOP increased.
However, in mice that are unable to secret SPARC, TGFß2 cannot increase the IOP. This was an unexpected finding and highly important—the holy grail of glaucoma.
The SPARC is one of the few proteins that regulate IOP. Doctors are starting to uncover these pathways with the hopes of interrupting them and thereby interrupting the disease pathophysiology.
The bottom line is that they discovered a protein that regulates IOP normally and is likely critical to the pathophysiology of POAG. This discovery will ultimately allow intervention at the molecular level.
(1).jpg)