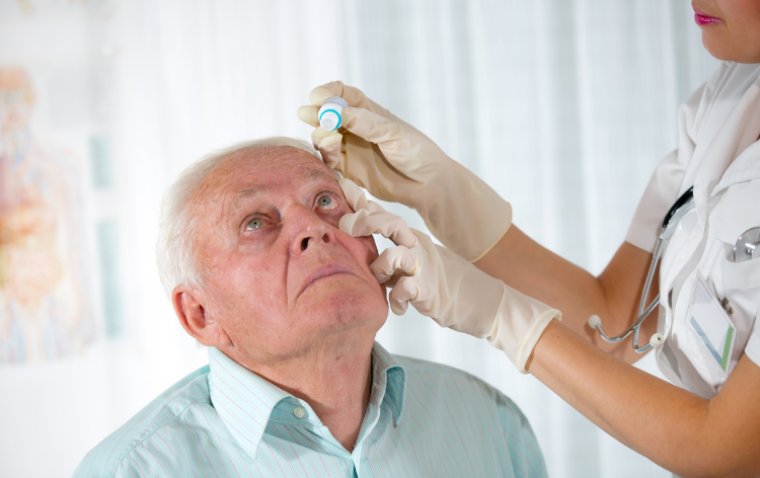
Primary Open Angle Glaucoma (POAG)
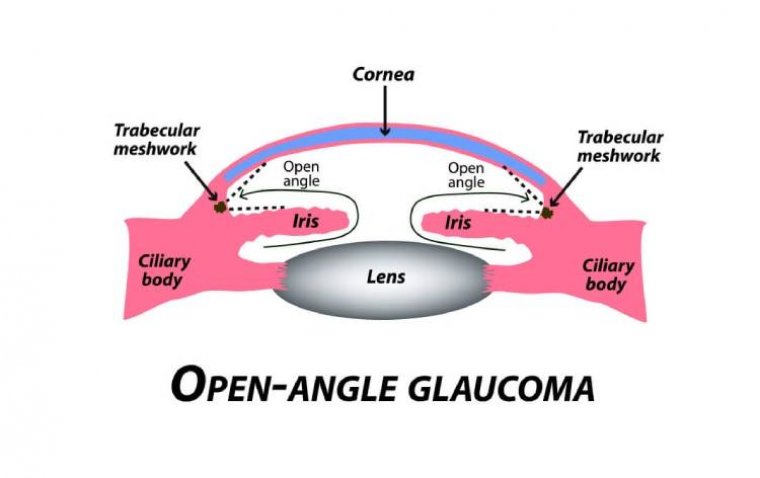
A subtype of glaucomas known as primary open angle glaucoma (POAG) is characterized by an open, seemingly normal anterior chamber angle and elevated intraocular pressure (IOP), with no additional underlying disease. Secondary glaucoma is a condition where the elevated IOP has a known underlying etiology.
Because the appropriate volume of fluid cannot drain out of the eye, the inner eye pressure (also known as intraocular pressure or IOP) increases. The drainage canal openings are visible and ought to be functioning properly in open-angle glaucoma.
Similar to a clogged pipe underneath the drain in a sink, the clogging issue develops farther inside the drainage canals.
The majority of folks don't exhibit any symptoms or warning signals. Undiagnosed and untreated open-angle glaucoma can result in a progressive loss of vision.
This particular form of glaucoma develops gradually, sometimes without any obvious vision loss for many years. When treated early on, it typically responds favorably to medicine.
Over two-thirds of instances of glaucoma are due to primary open-angle glaucoma (POAG), which is predicted to affect 3% of individuals over the age of 40 worldwide in 2020.
The National Institute for Health and Care Excellence (NICE) suggests a generic prostaglandin analogue as first-line therapy for chronic open-angle glaucoma. If a patient cannot tolerate their present medication, they should be provided medications from a different class or preservative-free eye drops.
After trying drugs from two therapeutic classes, practitioners are recommended to consider offering surgery with pharmacological augmentation as indicated or laser trabeculoplasty.
SLT as a First-line Option for Newly Diagnosed Glaucoma or Ocular Hypertension
The results of a partial review of the current NICE glaucoma management guidelines, which will compare the efficacy of selective laser trabeculoplasty (SLT) as a first-line treatment to eyedrops that lower intraocular pressure (IOP) for ocular hypertension (OHT) or chronic OAG, are expected in January 2022.
This comes after data from the LiGHT clinical trial showed that SLT is just as effective as eye drops when used as a first-line therapy for glaucoma or OHT in terms of quality of life (QoL) and clinical outcomes.
Similar health-related QoL scores were seen for the SLT and eye drop therapy groups in the LiGHT experiment. Visual acuity, IOPs, and mean deviations of the visual field were endpoints that were equivalent for both treatment arms.
At three years, around 75% of SLT patients had no drops, although about 25% did not. SLT-eyes were within goal IOP at 93.0% of visits, while the majority of drop eyes (91.3%) were also in this situation.
Although the odds are slim, topical treatment may have higher risks of glaucomatous degeneration (5.8% vs. 3.8%) and necessitating trabeculectomy/cataract surgery.
Topical therapy is a suitable primary therapy when compared to SLT, with no discernible difference in measured health-related QoL, even though SLT may be a more affordable alternative to drops.
The fact that SLT does not provide a rapid reduction in IOP is another concern. Out of 16,379 individuals evaluated for eligibility, 15,661 (94.5%) did not match the inclusion criteria for enrollment in the LiGHT trial, taking into account patient demographics seen in regular clinical practice.
While the majority of patients initially responded to SLT, a retrospective observational study (n=831) from five UK teaching centers indicated that most patients failed within one year — treatment success was recorded in 70%, 45%, and 27% of eyes at 6, 12 and 24 months post-SLT, respectively.
Higher baseline IOP was strongly associated with treatment success (hazard ratio [HR], 0.67 for baseline IOP >21mmHg vs. ≤21mmHg; 95% CI: 0.57–0.80; P<0.001).
Consultation Guideline for Angle-Closure Glaucoma
The Royal College of Ophthalmologists and the guideline development group are now reviewing the function of laser peripheral iridotomy (LPI) as part of a new draft clinical guideline on the management of angle-closure glaucoma.
Patients with restricted or blocked drainage angles are given prophylactic LPI by about 75% of UK ophthalmic doctors. However, there is little data to support the use of LPI as a prophylactic treatment in individuals who are asymptomatic and have never had a documented pressure rise.
This was supported by a randomised controlled experiment that demonstrated the minimal benefit of prophylactic LPI and came to the conclusion that widespread prophylactic LSI for PAC suspected was not advised.
Phacoemulsification clear lens extraction (phaco/CLE) is strongly advised as the definitive solution for PAC with IOP >30mmHg and PACG for the therapy of primary angle-closure glaucoma (PACG) in the draft clinical guideline [4].
The RCOphth advises against using preventive LPI on a large scale and on a regular basis in the NHS. A group of people known as PACS-PLUS who have additional risk factors may be appropriate for preventive LPI.
When acute (symptomatic) angle-closure occurs, prompt LPI is advised for all afflicted and contralateral eyes.
Advanced Glaucoma
The multicenter, randomized Treatment of Advanced Glaucoma Study (TAGS) trial, carried out in 27 centers throughout the UK, is a significant accomplishment because it offers the first concrete evidence of how patients with advanced glaucoma respond to primary trabeculectomy versus primary medical treatment.
Using the Hodapp classification of glaucoma severity, advanced disease was categorized according to the "severe" category of visual function (VF) loss.
There was no difference between treatment arms in the primary outcome, as determined by the Visual Function Questionnaire-25, at the 24-month follow-up (VFQ-25).
In terms of secondary outcomes, trabeculectomy required significantly fewer topical drugs to manage IOP than primary medication did at all time periods during the study.
At two years, the mean spectral domain (SD) IOP in the trabeculectomy and medical management groups was 12.4(4.7)mmHg and 15.1(4.8)mmHg, respectively. The authors stated that this more significant and long-lasting reduction in IOP (3-4mmHg) is expected to lead to superior visual field preservation over the course of a patient's lifetime.
Surgical Treatment: is Fear of ‘Wipe-Out’ Supported by Current Evidence?
According to NICE guidelines, persons with advanced OAG should be offered surgery together with any necessary pharmaceutical augmentation, as well as information on the risks and advantages of the procedure.
Because of perceived high-risk surgical consequences associated with trabeculectomy, including risks of blindness from bleb-related endophthalmitis and "wipe-out" right after surgery, clinicians frequently hesitate to do primary surgery for severe glaucoma.
After filtering surgery in eyes with severe glaucoma, the so-called "wipe-out" syndrome describes sudden, catastrophic visual loss that cannot be explained. At the 2021 RCOphth Virtual Annual Congress, Mr. Anthony King of Nottingham University Hospital, UK, presented on this subject during the Glaucoma Subspecialty Day.
The incidence and causation of visual acuity loss within three months following trabeculectomy were examined in a research by Costa et al. Four (0.95%) of 508 individuals experienced irreversible loss of central vision shortly after trabeculectomy.
Patients who were older, had macular splitting in the visual field before to surgery, and had significant hypotony (IOP 2 mmHg) on the first postoperative day (P=0.0246) were more likely to undergo "wipe-out."
Trabeculectomy with mitomycin C successfully lowers IOP in patients with advanced glaucoma over the short to medium term, according to an analysis of surgical outcome databases. Between one and seven years after trabeculectomy, the mean IOP of the 103 individuals included in the investigation was between 11.3 and 13.3mmHg.
85.2% of students at year five had IOPs under 16 mmHg. The majority of the twenty-eight individuals who reported a significant drop in acuity (2 lines of Snellen) did not have filtering surgery.
In a case series of 21 end-stage glaucoma patients who were monitored for three months following filtering surgery, IOP was effectively decreased and vision was retained without any instances of the "wipe-out" phenomena.
Early vision loss following glaucoma surgery in eyes with split fixation was prospectively evaluated, and it was discovered that early visual loss in advanced glaucoma is uncommon and typically results from reversible causes.
In this interventional group, there was no loss of central vision in any of the eyes. There were no surgically-induced changes in BCVA according to a clinical cohort research and meta-analysis examining the impact of glaucoma surgery (Baerveldt implant or trabeculectomy) on visual performance.
The scientists came to the conclusion that, after around 1.5 years, the benefit (long-term preservation of the visual field) of glaucoma surgery outweighed the cost (related loss of visual function).
According to the TAGS study, there was no sudden, inexplicable loss of vision following surgery, which excludes the possibility of a "wipe-out."
The trabeculectomy procedure did not result in significant vision loss, but two patients—one in each research arm—developed endophthalmitis. These patients received intravitreal antibiotic treatment and experienced good visual recovery.
The majority of reports of "wipe-out" after glaucoma surgery come from retrospective studies, according to Mr. King, and the phenomena is described differently in each study. However, the reported figures are small.
No recent prospective studies of individuals with advanced glaucoma have revealed catastrophic inexplicable vision loss following straightforward trabeculectomy surgery. Mr. King noted that the "wipe-out" occurrence following filtering operation is quite uncommon.
MIGS Devices: Evidence-Based Momentum
A review of glaucoma surgical procedures in the UK after the COVID-19 pandemic started indicates that trabeculectomy is being done less frequently.
Although trabeculectomy was the preferred surgery for the majority of glaucoma experts (87%, 61/70), conventional diode laser, glaucoma drainage devices, deep sclerectomy, and Preserflo microshunt appear to be preferred alternatives (Santen).
The iStent inject (Glaukos) and ab externo devices XEN 45 (AbbVie/Allergan) were the two minimally invasive glaucoma surgery (MIGS) procedures that were performed the most frequently in the 12 months prior to COVID-19, and a sizable percentage of respondents (73%) reported using alternative surgical techniques as part of their glaucoma toolbox.
Only ab interno non-bleb forming operations, according to European Glaucoma Society guidelines, are considered minimally invasive glaucoma surgeries.
In patients with mild-to-moderate POAG with cataract, implanting the iStent inject device at the time of phacoemulsification is a safe and effective way to lower IOP and the requirement for glaucoma drugs.
The pivotal trial of the iStent inject trabecular micro-bypass in POAG and cataract showed significant IOP reductions over the course of two years, regardless of the baseline level of diurnal IOP or medication load.
The iStent inject may be useful in more complicated medical circumstances because intraocular pressure reductions rose with higher baseline IOP and remained steady across all preoperative drug doses.
Surgery outcomes are positively correlated with device protrusion within the anterior chamber, according to a prospective analysis of outcomes at 12 months in patients implanted with two iStent inject devices in combination with phacoemulsification. This finding suggests that Schlemm canal dilatation has a positive prognostic value.
The analysis revealed that the iStent inject devices are immobile for the first year following implantation. In 58.98% of operated eyes, IOP 18mmHg was attained without medication.
According to a Cochrane review of ab interno trabecular bypass surgery using the Hydrus Schlemm's canal microstent (Ivantis, Inc.) for OAG, there is moderate-certainty evidence that, when combined with cataract surgery, as opposed to when combined with cataract surgery alone, the Hydrus microstent likely increases the proportion of participants who do not need IOP-lowering medication and may further lower IOP at short- and medium-term follow-up.
The combination of cataract surgery and Hydrus microstent placement for mild to moderate POAG is safe, more efficient in lowering IOP with fewer medications, and less likely to lead to additional incisional glaucoma filtration surgery than cataract surgery alone, according to published three-year results from HORIZON.
Data collected over a five-year period showed a significant decrease in the need for invasive filtration surgery (cumulative probability of reoperation 2.5% with cataract surgery and Hydrus implant vs. 6.4% with cataract surgery alone) and subsequent glaucoma medication (65% medication free five years post-implant).
The COMPARE prospective randomised trial's 12-month findings show standalone MIGS with the Hydrus microstent led to a higher surgical success rate and required fewer medications than the 2-iStent procedure. This study was the first to directly compare the efficacy of various MIGS devices for use in glaucoma management without concurrent cataract surgery.
Two eyes in the 2-iStent group underwent secondary glaucoma surgery, whereas none of the Hydrus eyes did. Alcon announced in November 2021 that it intended to purchase Ivantis, Inc. and its Hydrus microstent for an initial $475 million payment. The agreement reinforces Alcon's worldwide ophthalmology portfolio and reaffirms its dedication to "the surgical glaucoma space."
The Preserflo microshunt implant has been found to be beneficial in treating glaucoma, according to study results.
Through month 12, a retrospective, multicenter study conducted in Europe found that the mean IOP had decreased significantly from 25.1 6.5 mmHg at baseline to 14.1 3.4 mmHg (P 0.0001), and preoperative medication use had decreased significantly from 3.0 1.0 medications to 0.77 0.95 medications (P 0.001).
Preserflo microshunt and trabeculectomy were both equally effective and safe in decreasing mean diurnal IOP at six months, according to a comparative outcomes analysis of a cohort of POAG patients (n=52). An extension trial (n=23) showed sustained decreases in mean IOP and medication use for up to five years after the placement of the Preserflo microshunt.
In eyes with refractory glaucoma, the PAUL glaucoma drainage device (Advanced Ophthalmic Innovations) exhibits comparable efficacy to other existing implants.
The PAUL glaucoma implant significantly lowers IOP and medication use, with few intraoperative and postoperative problems, according to early outcome data from Manchester Royal Eye Hospital.
Nine cases (9.3%) were deemed failures among a consecutive cohort of 99 eyes of 97 patients who underwent surgery with the PAUL glaucoma implant (between February 2019 and May 2020, under the supervision of five consultant surgeons). Of these, six cases had IOP reductions of less than 20% from baseline and three cases had IOPs greater than 21 mmHg.
Preoperative mean IOP was 28.1 9.0 mmHg on average, and at six months it was 13.6 4.7 mmHg. The mean change in the number of drugs was a decrease of 2.38 1.48 (n=52) and the mean IOP was 13.3 4.4 at one year.
There were two hypotonic cases. Using a 6/0 Prolene® intraluminal stent without the use of viscoelastic or Vicryl® overtie allowed for fast drainage and a more predictable IOP outcome during the first few weeks.
Novel-Acting Medical Treatment Netarsudil
Aerie Pharmaceuticals' Rhokiinsa, a powerful inhibitor of the Rho kinase and norepinephrine transporter, works by boosting trabecular outflow, reducing aqueous generation, and perhaps lowering episcleral venous pressure.
Fixed-combination netarsudil-latanoprost (Roclanda, Aerie Pharmaceuticals) got marketing authorization from the UK Medicines and Healthcare products Regulatory Agency (MHRA) in April 2021 after being given approval by the European Commission in January 2021.
It is intended to lower increased IOP in adult patients with POAG or OHT when a prostaglandin analogue or netarsudil alone does not sufficiently lower IOP.
In two major investigations, IOP reductions in Roclanda-treated patients were statistically superior to those attained by netarsudil and latanoprost monotherapy and were meaningful and therapeutically relevant.
Treatment with Roclanda demonstrated non-inferiority to fixed-combination bimatoprost and timolol (Ganfort, Allergan) across nine of nine timepoints over 90 days in Mercury 3, a phase 3b non-inferiority trial, with an average IOP decrease from baseline of about 37%.
(1).jpg)