Pandorum Technologies Secures $11 Million for Corneal Blindness Treatment
Pandorum Technologies, a pioneering startup in the field of Tissue Engineering and Regenerative Medicine, has successfully closed a pre-Series B funding round, raising $11 million. This significant investment will accelerate the development of its product, Kuragenx ("Liquid Cornea"), aimed at treating corneal blindness.
Investment Boost from Prominent Entities
The funds will also support the advancement of the company's tunable technology platform, which has shown promising regenerative capabilities for various tissues, including liver, lung, and neuronal tissues. The investment round saw participation from notable figures and entities such as Ashish Kacholia, Everest Finance Investment, Acebright Pharma, and Bandana Kankani's syndicate. They join existing investors Sunil Kant Munjal and the Indian Angel Network in backing the innovative endeavors of Pandorum Technologies.
Kuragenx: A New Horizon in Regenerative Medicine
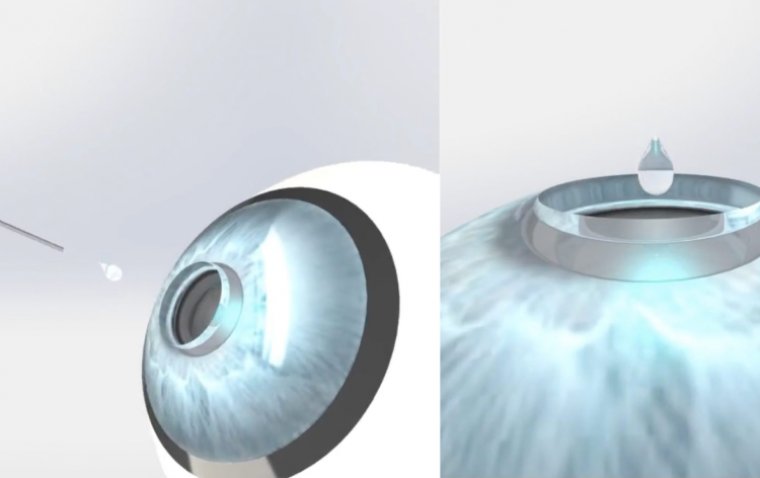
Co-Founders Arun Chandru and Dr. Tuhin Bhowmick are at the helm of the company, driving forward their mission to address unmet clinical needs through advanced therapeutics. "We are thrilled to have the support of the notable investors, including Mr. Ashish Kacholia, as we continue to innovate and develop therapeutics to restore healthy tissue functions, in order to help the patients suffering from unmet clinical needs," Dr. Bhowmick expressed. He further elaborated on the flagship product, Kuragenx, highlighting its unique approach: "Our flagship product Kuragenx combines proprietary biomaterials with regenerative nanotherapy, guiding the formation of a functional corneal tissue to restore vision. This is a first-in-class approach that can truly blur the line between treatment and cure."
Kuragenx stands out for its ability to promote scarless regeneration of corneal tissue, a promising feat demonstrated in pre-clinical studies. Recognizing its potential, the FDA awarded Kuragenx the Orphan Drug Designation for treating neurotrophic keratitis in 2023. With this funding, Pandorum is set to embark on clinical manufacturing process and IND enabling studies, with the first patient dosing anticipated in 2025, pending regulatory approvals.
Pandorum Technologies' progress marks a significant milestone in the quest to treat corneal blindness, offering hope to patients worldwide with its innovative approach to regenerative medicine.
About Corneal Blindness
Corneal blindness is a significant global health concern, characterized by the loss of vision due to damage or disease affecting the cornea, the clear front surface of the eye. It can result from various conditions, including infections, injuries, genetic disorders, and degenerative diseases like keratoconus. Corneal blindness is particularly troubling because the cornea is crucial for focusing light onto the retina, enabling us to see clearly.
In many cases, corneal blindness can be treated through surgical interventions, such as corneal transplantation, where damaged corneal tissue is replaced with healthy tissue from a donor. However, challenges such as a shortage of donor corneas and the risk of rejection complicate treatment options. Advances in tissue engineering and regenerative medicine are opening new avenues for treatment, potentially offering innovative solutions to restore vision in individuals affected by corneal blindness.
Reference:
(1).jpg)