NIH Researchers Use AI to Sharpen Standard Ophthalmoscopes for High-Resolution Retinal Imaging
Scientists at the National Institutes of Health (NIH) have developed an artificial intelligence (AI) system that transforms standard eye clinic imaging devices into powerful tools capable of visualizing individual retinal cells. The breakthrough, published in Communications Medicine, enables image quality comparable to advanced experimental systems, but without the need for specialized equipment or expertise.
Enhancing Standard Imaging Through AI
Scanning laser ophthalmoscopes are widely used in clinical ophthalmology to view the retina. However, they traditionally only capture tissue-level features such as blood vessels, lesions, and the optic nerve head. In contrast, adaptive optics-enabled ophthalmoscopes, which are still largely used in research settings, can resolve structures at the cellular level.
To bridge this technological gap, Dr. Johnny Tam, investigator at the NIH's National Eye Institute, and his team created a custom AI-based enhancement system that significantly sharpens images acquired from standard ophthalmoscopes.
“AI potentially puts next-generation imaging in the hands of standard eye clinics,” said Dr. Tam. “It’s like adding a high-resolution lens to a basic camera.”
Training AI to Reveal the Invisible
The research team focused on imaging the retinal pigment epithelium (RPE)—a layer of cells beneath the photoreceptors that plays a vital role in retinal health and is affected early in macular degenerations such as:
• Age-related macular degeneration (AMD)
• Stargardt disease
• Vitelliform macular dystrophy
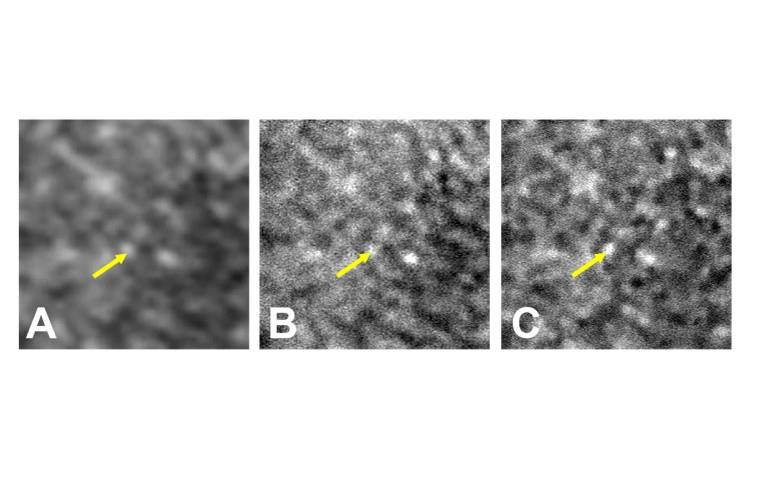
To train the AI model, researchers first categorized over 1,400 adaptive optics ophthalmoscopy images as poor, moderate, or good quality. They then provided the system with corresponding standard ophthalmoscopy images from the same retinal regions.
Once trained, the AI system enhanced the clarity of standard images by up to eightfold, successfully revealing cellular-level details that are otherwise difficult to observe in routine clinical practice.
“Our system used what it learned from adaptive optics imaging to digitally enhance the clarity of standard ophthalmoscopy,” said Dr. Tam. “It’s important to note that the AI is not fabricating new data—it’s clarifying what is already there.”
Boosting Retinal Imaging with ICG and AI
To further enhance contrast and highlight RPE cells, the team employed indocyanine green (ICG) dye, a contrast agent commonly used in eye clinics for vascular imaging. The use of ICG enabled clearer visualization of the RPE, especially when combined with the AI system.
“Our ICG imaging strategy allows RPE cells to be quickly and routinely assessed in the clinic,” said Dr. Joanne Li, first author of the report and biomedical engineer in Dr. Tam’s lab. “With AI, high-quality RPE imaging can be done in seconds using standard equipment.”
Clinical Implications: Making the Invisible Visible
RPE dysfunction is an early marker in many blinding eye diseases. However, conventional tools have made it difficult to assess RPE health without specialized research-grade instruments. This AI-powered enhancement strategy allows:
• Routine visualization of RPE cells
• Early detection of degenerative retinal conditions
• Monitoring of treatment response
By combining AI with standard imaging devices and clinically approved dyes, the NIH team has made advanced retinal diagnostics more accessible, offering a practical, scalable solution for everyday clinical settings.
Reference:
Joanne Li et al, Artificial intelligence assisted clinical fluorescence imaging achieves in vivo cellular resolution comparable to adaptive optics ophthalmoscopy, Communications Medicine (2025). DOI: 10.1038/s43856-025-00803-z
(1).jpg)