Intalight Secures CE Mark for DREAM OCT Platform, Clears Path for Commercialization in Europe
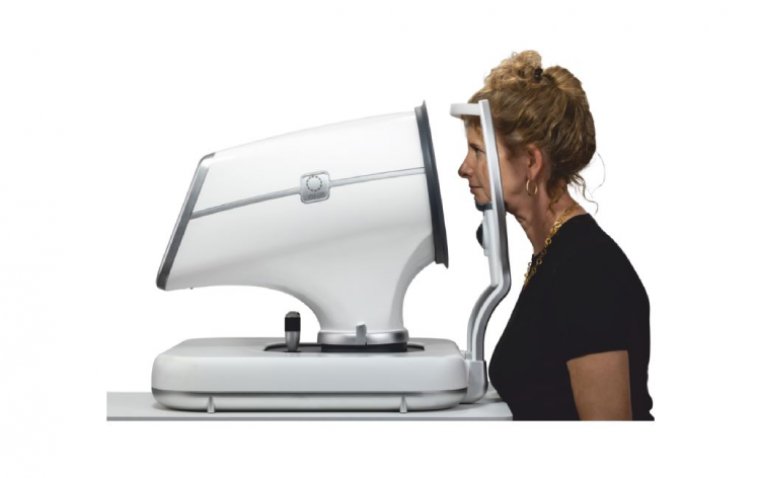
Intalight has announced that it has received CE Mark approval for its next-generation DREAM OCT platform, authorizing the commercialization of the technology across the European Union. The DREAM OCT system represents a significant advancement in optical coherence tomography (OCT), designed to support both clinical and research applications in ophthalmology.
The CE mark confirms that the DREAM OCT platform complies with EU safety, health, and environmental protection standards, allowing for broad European market access.
“Intalight is incredibly proud to receive CE mark approval in Europe for our DREAM OCT,” said Shawn Peng, Chairman and Founder of Intalight. “This achievement allows us to provide ophthalmologists in Europe with state-of-the-art technology that delivers improved results for their patients.”
DREAM OCT: Deep, Rapid, Extensive, Accurate, Multimodal
The acronym DREAM reflects the platform’s defining features:
• Deep imaging depth
• Rapid sweeping speed
• Extensive scan range
• Accurate results
• Multimodal imaging capabilities
DREAM OCT offers a 130° OCTA image from a single ultrawide scan, providing detailed visualization of the choroid, retina, and vitreous space through its 12 mm super-depth swept source OCT. Additionally, the system can scan 16.2 mm (in air) anteriorly, capturing the entire anterior segment—from the cornea to the anterior vitreous—in a single pass.
“Over the past few years, we’ve heard from eye care professionals that they need a solution that gets them over the imaging finish line with speed, accuracy, and depth,” said Bing Li, CEO and Co-Founder of Intalight. “DREAM OCT delivers a full set of imaging modalities for the most challenging clinical and research applications for the retina and outperforms everything else HCPs know.”
Backed by Research and Global Partnerships
Intalight reports that the DREAM OCT platform has already contributed to over 160 peer-reviewed publications, underscoring its clinical value and reliability. The company is actively collaborating with leading retina academic centers and private practices across the United States, Europe, and Asia.
Looking ahead, Intalight plans to make the DREAM OCT platform available in the U.S. market, pending regulatory approval.
(1).jpg)