Horizon’s Tepezza Improves Proptosis in Patients With Chronic TED
Horizon Therapeutics has announced positive and statistically significant topline results from its randomized, double-masked, placebo-controlled Phase 4 clinical trial evaluating TEPEZZA for the treatment of adults with chronic thyroid eye disease (TED) and low CAS, a measure of disease activity.
The clinical trial data supports the efficacy of TEPEZZA in a broad spectrum of TED patients, regardless of disease activity or duration, with a well-established safety profile. TEPEZZA is the first and only medicine approved by the FDA for the treatment of TED, a serious, progressive, debilitating, and potentially vision-threatening rare autoimmune disease.
In the Phase 4 trial, patients diagnosed with TED for a duration of two to 10 years (mean duration of 5.2 years; SD 1.77) and low levels of disease activity (mean CAS of 0.4; SD 0.49) were evaluated. This is in contrast to the prior pivotal trials (Phase 2 and 3) that were used for the original FDA approval of TEPEZZA, which evaluated patients with a disease duration of nine months or less and higher levels of disease activity.
“We are thrilled with the topline results, which reinforce that TEPEZZA significantly reduces proptosis in people living with Thyroid Eye Disease, regardless of their disease activity or duration, and underscores what we learned from our initial trials and what we have seen through more than three years of real-world use of TEPEZZA,” said Elizabeth H.Z. Thompson, Ph.D., executive vice president, research and development, Horizon.
“With TEPEZZA, physicians have a medicine that can be used in a broad range of Thyroid Eye Disease patients, including those with long-duration disease of more than 5 years on average in this trial, which is important because we know the negative impact of the disease can be significant across all types of Thyroid Eye Disease patients. We look forward to discussing these data with the FDA to determine our next steps,” she added.
Topline Data Overview
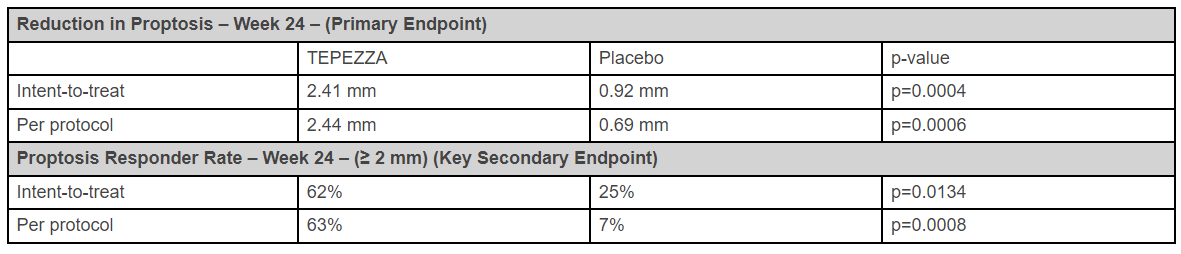
At Week 24, the topline data, analyzed using the pre-specified primary analysis method (intent-to-treat), showed that the primary endpoint was met. Patients who were treated with TEPEZZA demonstrated a statistically significant reduction in proptosis from baseline compared to those who received placebo. Moreover, in the pre-specified per-protocol analysis, the differences between patients treated with TEPEZZA and placebo further increased.
“Given this new and positive clinical evidence in patients with long-duration Thyroid Eye Disease and low CAS, it is important for physicians to thoroughly assess all of their Thyroid Eye Disease patients to determine whether TEPEZZA might be an option,” said Raymond Douglas, M.D., Ph.D., the trial’s principal investigator and director of the Orbital and Thyroid Eye Disease Program, Cedars-Sinai Medical Center in Los Angeles. “It is important to specifically ask patients if their symptoms are interfering with their ability to work, socialize and go about daily activities. These conversations can help physicians uncover the true burden of the disease and need for treatment, regardless of how much inflammation they have behind the eye or how long they have been living with the disease.”
(1).jpg)