Vyjuvek Eye Drop Restores Vision in Teen with Rare Genetic Condition
Krystal Biotech's topical gene therapy Vyjuvek (beremagene-geperpavec) has successfully restored the vision of a 13-year-old boy suffering from the rare genetic condition dystrophic epidermolysis bullosa (DEB), which scarred his eyes.
The groundbreaking case, detailed in a recent publication in The New England Journal of Medicine, showcases the potential of Vyjuvek in treating ocular manifestations of DEB.
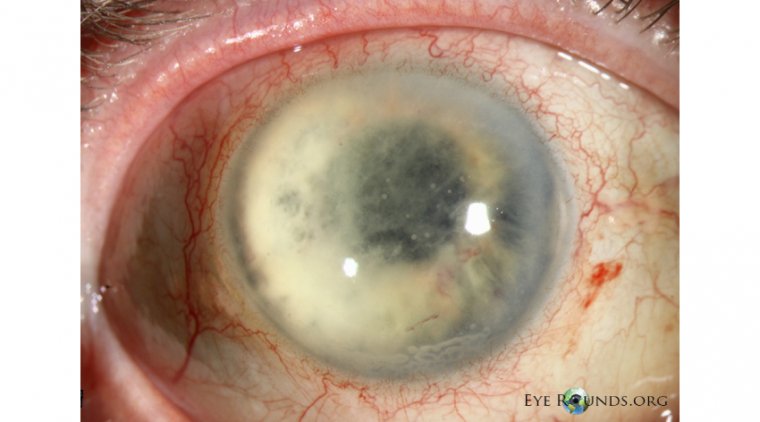
The patient, identified only as Antonio in reports by the Associated Press, presented with chronic wounds and webbed hands and fingers, alongside compromised vision with a best-corrected visual acuity of 20/40 in the right eye and 20/80 in the left eye. Examination revealed scar tissue in both eyes, indicative of the severity of his condition.
Despite undergoing multiple aggressive interventions, including surgeries and various treatments, Antonio's condition continued to worsen, with lesions recurring even after initial healing. However, his participation in a Phase III trial of Vyjuvek in 2020 proved to be a turning point. Significant healing of his wounds was observed, prompting further exploration of Vyjuvek's potential for ocular surface involvement in DEB.
After the FDA cleared an Investigational New Drug application for compassionate use of Vyjuvek, Antonio underwent another round of surgery followed by topical administration of the gene therapy as eyedrops. The results were astounding—within three months and after 19 Vyjuvek doses, Antonio's corneal epithelium fully healed, as confirmed by clinical evaluation and imaging techniques. At eight months, assessments revealed complete epithelial healing, with no signs of scarring or recurrence. Notably, his visual acuity improved to 20/25, demonstrating the efficacy of Vyjuvek in restoring vision.
Despite one surgery-related serious adverse event flagged in the NEJM paper, Vyjuvek treatment continued without interruption. The researchers emphasized the importance of further investigation into Vyjuvek's potential for DEB patients with ocular surface involvement, citing Antonio's case as compelling evidence of its efficacy in promoting corneal epithelial healing.
Vyjuvek works by delivering two functional copies of the COL7A1 gene, which is defective in DEB due to genetic mutations, resulting in dysfunctional type VII collagen proteins. DEB is characterized by extremely fragile skin prone to wounding and tearing, with ocular manifestations posing additional challenges for patients.
In May 2023, Vyjuvek made history as the first-ever redosable gene therapy approved by the FDA for the treatment of DEB in patients aged six months and older. This landmark approval underscores the potential of gene therapy in addressing complex genetic conditions and offering hope to patients like Antonio facing debilitating manifestations of DEB.
Reference
https://www.nejm.org/doi/full/10.1056/NEJMoa2301244?query=featured_home
*Stay in the loop and make sure not to miss real-time breaking news about ophthalmology. Join our community by subscribing to OBN newsletter now, and get weekly updates.
(1).jpg)