Empagliflozin Linked to Reduced Progression of Diabetic Retinopathy
A study led by Brigham and Women's Hospital has revealed that empagliflozin (Jardiance), a sodium-glucose cotransporter-2 inhibitor (SGLT2i), may significantly reduce the risk of diabetic retinopathy (DR) progression in patients with nonproliferative diabetic retinopathy (NPDR). However, the study found no evidence that empagliflozin prevents the onset of new NPDR cases.
Insights into Diabetic Retinopathy
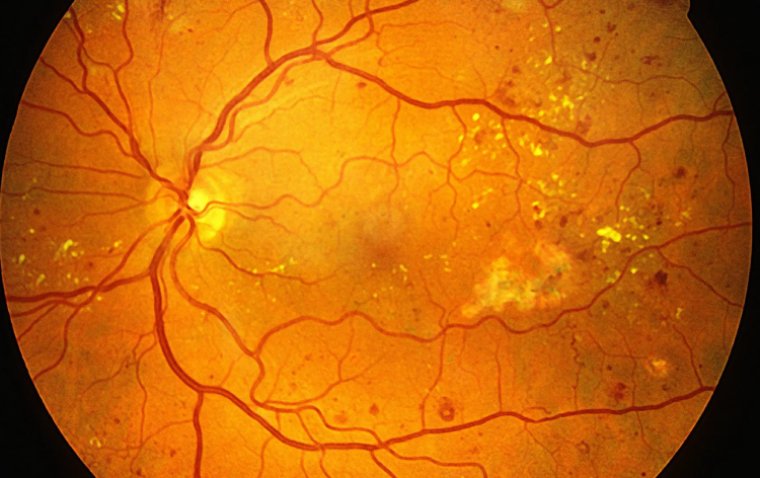
Diabetic retinopathy is one of the most common complications of type 2 diabetes, affecting approximately 26% of patients in the United States in 2021. It is a leading cause of irreversible vision loss among working-age adults, with prevalence expected to rise in the coming decades.
Empagliflozin, originally developed to manage blood glucose levels, has demonstrated cardiovascular and renal benefits in previous clinical trials. Its impact on diabetic retinopathy progression, however, has remained unclear until now.
Study Design and Key Findings
The cohort study, titled "Empagliflozin and the Risk of Retinopathy in Patients With Type 2 Diabetes," was published in JAMA Ophthalmology. Researchers analyzed insurance claims data from Medicare and two major commercial health plans over a five-year pre-COVID period.
Study Groups
The study involved two matched patient cohorts:
• 34,239 pairs to evaluate the risk of developing new NPDR.
• 7,831 pairs to examine DR progression in those with preexisting NPDR.
Cohorts were monitored for an average of eight months after initiating empagliflozin or dipeptidyl peptidase-4 inhibitors (DPP4i) therapy.
Findings
1. Onset of New NPDR:
• No significant difference was observed between the two groups.
• Empagliflozin users experienced 741 events versus 712 events in the DPP4i group (HR = 1.04).
2. Progression of Preexisting DR:
• Empagliflozin users had fewer events (158) compared to the DPP4i group (201).
• This corresponds to a 22% reduction in risk (HR = 0.78).
• Cumulative incidence curves favored empagliflozin for DR progression early in the follow-up period.
The findings are consistent with prior analyses from the EMPA-REG OUTCOME trial, which also reported a 22% reduction in DR-related events with empagliflozin compared to placebo.
Clinical Implications
While empagliflozin does not appear to prevent new NPDR onset, its association with reduced DR progression in patients with preexisting NPDR suggests it could be a valuable therapeutic option for managing diabetic retinopathy in these individuals.
Expert Commentary
The study’s authors emphasize that further research is needed to explore:
• Long-term outcomes.
• Detailed patient data, including diabetes duration and baseline DR severity.
• Direct patient evaluations beyond claims data.
This investigation underscores the potential role of empagliflozin in slowing DR progression, offering hope for improved management of this sight-threatening condition.
Resource:
Helen Tesfaye et al, Empagliflozin and the Risk of Retinopathy in Patients With Type 2 Diabetes, JAMA Ophthalmology (2024). DOI: 10.1001/jamaophthalmol.2024.5219
(1).jpg)