Atsena Therapeutics Publishes 12-Month Data on ATSN-101 for LCA1
Atsena Therapeutics announced that 12-month safety and efficacy data from its Phase I/II trial of ATSN-101, a gene therapy for Leber congenital amaurosis caused by GUCY2D mutations (LCA1), were published in The Lancet. ATSN-101 showed durable improvements in vision at the high dose and was well-tolerated in all patients.
Fifteen patients received subretinal injections of ATSN-101. High-dose subjects saw significant improvements in visual function, with a 100-fold increase in dark-adapted full-field stimulus test (FST). Ocular inflammation was mild and managed with steroid treatment.
Clinical Data and Safety
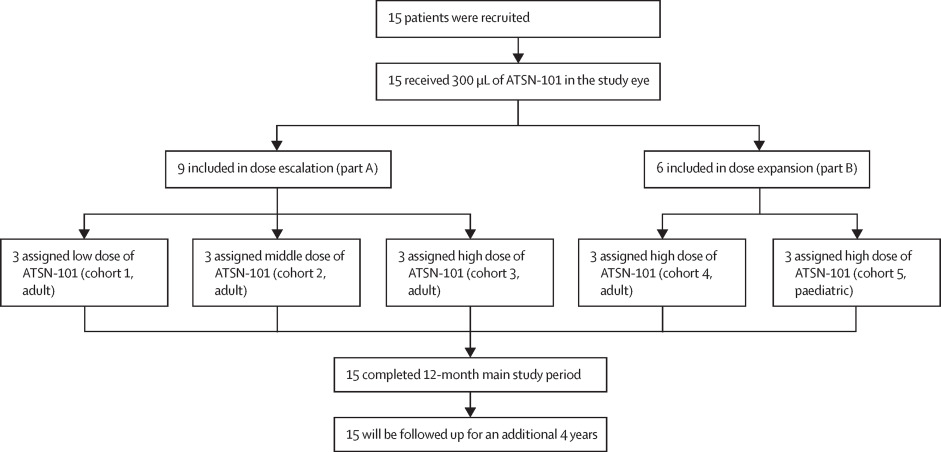
Trial Design
The Phase I/II trial evaluated the safety and preliminary efficacy of ATSN-101 in patients with genetically confirmed LCA1. The study involved a dose-escalation phase, where three adult cohorts (n=3 each) received ascending doses of 1.0E10 vg/eye (low dose), 3.0E10 vg/eye (mid dose), and 1.0E11 vg/eye (high dose). Following this, a dose expansion phase treated one adult and one pediatric cohort (n=3 each) at the high dose. All 15 patients received unilateral subretinal injections, with the primary endpoint being the incidence of treatment-emergent adverse events (TEAEs). Secondary endpoints included the full-field stimulus test (FST), best-corrected visual acuity (BCVA), and a multi-luminance mobility test (MLMT).
Among high-dose patients, improvements in FST were observed, with a mean change of 20.3 decibels, representing a 100-fold improvement. This improvement persisted through 12 months, with some patients achieving the maximum score in the MLMT. Modest improvements were also seen in BCVA, with an average improvement of -0.16 logMAR (equivalent to 8 letters) for high-dose subjects at 12 months post-treatment.
Safety Data
.jpg)
Outer retinal changes in a patient who received the high dose of ATSN-101
Adverse events reported in the study were primarily mild (91%) or moderate (9%) in severity, with no serious events directly linked to the ATSN-101 therapy. The majority of TEAEs were related to the surgical procedure, while any ocular inflammation was mild and effectively managed with steroids.
Kenji Fujita, MD, Chief Medical Officer of Atsena Therapeutics, noted, “The preliminary efficacy and robust safety data from our ongoing Phase I/II trial underscore the potential of ATSN-101 to be a first-in-class gene therapy for LCA1. It is encouraging that improvements in retinal sensitivity were observed as early as 28 days post-treatment and persisted over at least 12 months, highlighting the durability of our investigational gene therapy.”
Future Implications
LCA1 is an inherited retinal disorder caused by GUCY2D mutations, leading to severe vision impairment or blindness in infancy. With no approved treatments, ATSN-101 represents a significant step forward in the potential treatment of this condition. The results from this trial support further evaluation of ATSN-101 in a future randomized Phase III trial.
About Atsena Therapeutics
Atsena Therapeutics is a clinical-stage gene therapy company focused on developing treatments for inherited retinal diseases. Its lead program, ATSN-201, is in a Phase I/II trial for X-linked retinoschisis (XLRS), a genetic condition affecting boys and men. Another Phase I/II trial is evaluating ATSN-101 for Leber congenital amaurosis type 1 (LCA1), a leading cause of blindness in children. Atsena uses novel adeno-associated virus (AAV) technology to address challenges in treating retinal diseases.
(1).jpg)